Readers of this blog presumable like laboratory medicine. If you also like listening to podcasts, check out Lab Medicine’s podcast series on iTunes.
Tag: pathology
Hematopathology Case Study: A 45 Year Old Male with Mediastinal Mass
Case History
A 45 year old male underwent a chest MRA for aortic dilation due to his history of an aneurysmal aortic root. Upon imaging, an incidental anterior mediastinal mass was seen that measured 4.0 cm. In preparation for an upcoming cardiac surgery, the patient underwent a thymectomy with resection of the mass. The sample is a section from the mediastinal mass.
Diagnosis

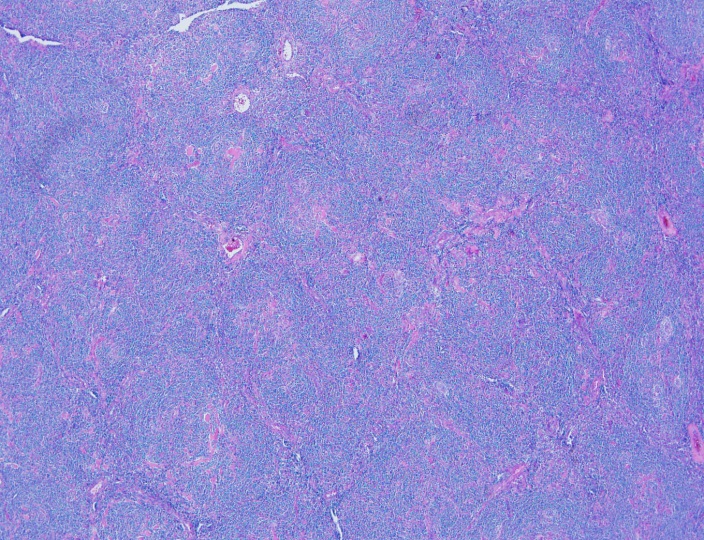

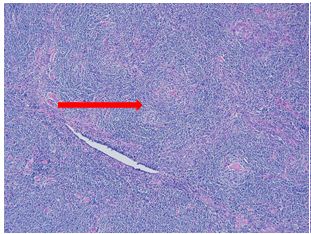
Sections show an enlarged lymph node with several follicles demonstrating atrophic-appearing germinal centers which are primarily composed of follicular dendritic cells. These areas are surrounded by expanded concentrically arranged mantle zones. Focal “twinning” of germinal centers is present. Additionally, prominent centrally placed hyalinized vessels are seen within the atrophic germinal centers giving rise to the “lollipop” appearance.
By immunohistochemistry, CD20 highlights B-cell rich follicles while CD3 and CD5 highlight abundant T-cells in the paracortical areas. CD10 is positive in the germinal centers while BCL2 is negative. CD21 highlights expanded follicular dendritic meshwork. CD138 is positive in a small population of plasma cells and are polytypic by kappa and lambda immunostaining. HHV8 is negative. MIB1 proliferation index is low while appropriately high in the reactive germinal centers.
Overall, taking the histologic and immunophenotypic findings together, the findings are in keeping with Castleman’s disease, hyaline vascular type. The reported clinical and radiographic reports suggest a unicentric variant.
Discussion
Castleman’s disease comes primarily in two varieties: localized or multicentric. The localized type is often classified as the hyaline vascular type (HVCD). Demographically, it’s a disease of young adults but can be found in many ages. The most common sites for involvement are the mediastinal and cervical lymph nodes.
The classic histologic findings of HVCD involve numerous regressed germinal centers with expanded mantle zones and a hypervascular interfollicular region. The germinal centers are predominantly follicular dendritic cells and endothelial cells. The mantle zone gives a concentric appearance, often being likened to an “onion skin” pattern. Blood vessels from the interfollicular area penetrate into the germinal center at right angles, giving rise to another food related identifier, “lollipop” follicles. A useful diagnostic tool is the presence of more than one germinal center within a single mantle zone.
The differential diagnosis of HVCD includes late stage HIV-associated lymphadenopathy, early stages AITL, follicular lymphoma, mantle cell lymphoma, and a nonspecific reactive lymphadenopathy. A history of HIV or diagnostic laboratory testing for HIV would exclude the first diagnosis. AITL usually presents histologically as a diffuse process but atypia in T-cells with clear cytoplasm that co-express CD10 and PD-1 outside of the germinal center are invariably present. EBER staining may reveal EBV positive B immunoblasts in early AITL, which would be absent in HVCD. The most challenging differential would include the mantle zone pattern of mantle cell lymphoma. Flow cytometry revealing a monotypic process with co-expression of cyclin D1 on IHC would further clarify the diagnosis.1
Overall, unicentric Castleman’s disease is usually of the hyaline vascular type. Surgical resection is usually curative in these cases with an excellent prognosis.2
References
- Jaffe, ES, Harris, NL, Vardiman, J, Campo, E, Arber, D. Hematopathology. Philadelphia: Elsevier Saunders, 2011. 1st ed.
- Ye, B, Gao, SG, Li, W et al. A retrospective study of unicentric and multicentric Castleman’s disease: a report of 52 patients. Med Oncol (2010) 27: 1171.

-Phillip Michaels, MD is a board certified anatomic and clinical pathologist who is a current hematopathology fellow at Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA. His research interests include molecular profiling of diffuse large B-cell lymphoma as well as pathology resident education, especially in hematopathology and molecular genetic pathology.
A Serious Aside
As an unscheduled post, I’d like to make a quick side note separate from public health, zika, and medical school. You may have seen a post I wrote last January about the potential stereotypes and stigmas we might face in laboratory medicine. But, just because we as laboratory professionals operate behind-the-scenes most of the time, we’re still healthcare professionals—and clinician burnout can affect any of us.
I recently watched a video of Dr. Zubin Damania, also known as “ZDoggMD,” a primary care physician and founder of Turntable Health in Las Vegas. He’s a brilliant and passionate doctor with great opinions and an even greater creative sense of humor. Among his many parodies, and “rounds” Q&A questions, ZDoggMD recently had a guest on one of his Facebook shows called “Against Medical Advice” to address the serious issue of suicide and depression in medicine. Janae Sharp was the guest on this episode speaking about her husband, John, a physician fresh into his residency who committed suicide. They go on to talk about her life after this tragedy and how if flipped her and their children’s’ lives upside down. Janae’s described John as a father, a writer, a musician, an idealist, who always wanted to become a doctor. My interest was definitely piqued by this—I tend not to miss most of Dr. Damania’s content—and this is something I’ve been hearing more and more about as my path through medical school continues. But, at one point in the interview my heart just stopped: John was a clinical pathologist. Too close to home, for me at least. I was admittedly surprised.
Pathologist’s don’t have that much stress to make depression and suicide part of that life, I thought. But that is a cold hard assumption. Depression affects so many people at large, and when you’re in healthcare it almost seems like a risk factor on top of issues one might be struggling with. Med school is touted as one of the hardest intellectually, physically, and emotionally grueling experiences you could go through—I will personally vouch for Dr. John and Dr. Damania’s statements about how much these experiences push you to your limits. No sleep, no recognition, no support, fear of failure, imposter syndrome, a wealth and breadth of knowledge that makes you feel like you’re drowning—not to mention that if you do ask for help you’re immediately “lesser” for doing so.
Video 1. ZDoggMD interviews Janae Sharp about her tragic loss, her husband John’s suicide, and the rampant problem of depression and burnout in medicine. Against Medical Advice, Dr. Damania.
Last month, I was fortunate enough to attend a grand rounds session at my current hospital about this very topic. Presented by Dr. Elisabeth Poorman, internal medicine attending physician, and clinical instructor at Harvard Medical School, who talked about how (because of stigmas) medical trainees don’t get the help they need. She demonstrated that prior to med school students are pretty much on-par with their peers with regard to depression. However, once medical school starts, those peers all plummet together as depression rates rise and fall dramatically throughout the various stages of their careers. (I’m just going to go ahead and vouch for this too.) Dr. Poorman shared several case studies that effectively conveyed just how hard it can be when it seems like you are a source of help for many, but no one is there to help you. Story and story recounted the same model of apparent—and often secretive—burnout which ultimately led to a decrease in the quality of care, and in some instances suicide. Dr. Poorman was also brave enough to share her own story. No stranger to depression, herself, it was something that she encountered first hand. She connected herself with this increasingly difficult picture of inadequate support for those of us spending our lives serving others.
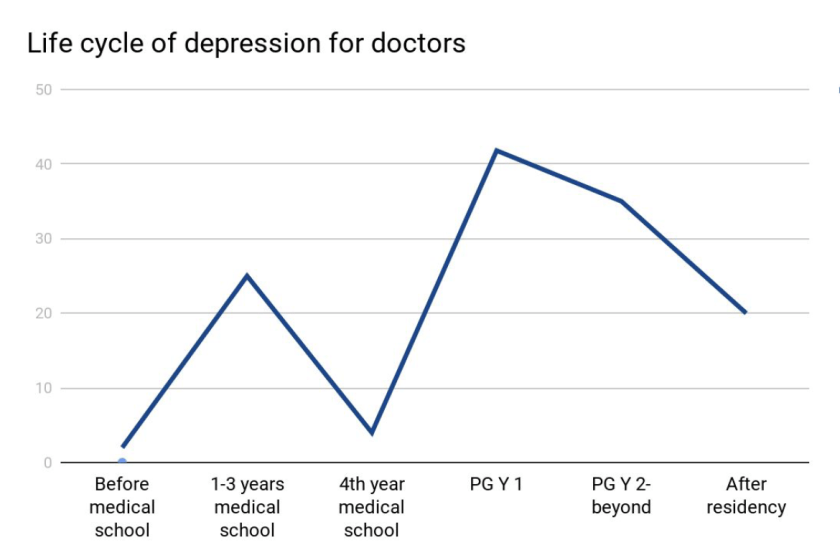
There are clear problems facing those of us in healthcare jobs. An ironic consequence, however, of modern scientific advancement is the “doubling time” of medical knowledge. While not necessarily a problem, this refers to the amount, depth, and scope of knowledge physicians and medical scientists are expected to master in order to effectively treat, make critical clinical decisions, and educate our patients. While in 1980 it took 7 years for all medical knowledge to double in volume, it only took 3.5 years in 2010, and in 2020 it’s expected to double every 73 days!1. The problems come as a result of this knowledge because more data means more to do. More time on the computer, higher critical responsibility, and less time to focus on your own mental health all lend themselves to a cyclic trap of burnout. Physicians commit suicide at a rate of 1.5 – 2.3 times higher than the average population.1
Physicians, nurses, clinical scientists, lab techs, administrators, phlebotomists, PCTs—we’re all over worked, under-supported, fall victim to emotional fatigue, and have some of the highest rates for depression, substance abuse, PTSD, and suicide.1 Sometimes, reports from Medscape or other entities will report that burnout is a phenomenon of specialty, hypothesizing that critical nature specialties have more depression than lesser ones2 (the assumption that a trauma surgeon might burn out before a hematopathologist). But truthfully, this is just part of the landscape for all providers. A May 2017 Medscape piece wrote “33% chose professional help, 27% self-care, 14% self-destructive behaviors, 10% nothing, 6% changed jobs, 5% self-prescribed medication, 4% other, 1% pray.”3
So I’m talking about this. To get your attention. So that people reading know they’re not alone. So that people with friends going through something can lend a hand. I’m talking about this. ZDoggMD is talking about this. Jamie Katuna, another prolific medical student advocate, is talking about this. Dr. Elisabeth Poorman is talking about this. This is definitely something we should come together to address and ultimately solve.
What will you do to help?
This was a heavy topic. So in a lighter spirit, I have to share this with all of my laboratory family. If you haven’t heard or seen Dr. Damania’s videos yet, this is the one for you:
Thanks! See you next time!
References
- Poorman, Elisabeth. “The Stigma We Live In: Why medical trainees don’t get the mental health care they need.” Cambridge Health Alliance, Harvard Medical School. Grand rounds presentation, Feb 2018. Bronx-Lebanon Hospital Center, New York, NY.
- Larkin, Mailynn. “Physician burnout takes a toll on U.S. patients.” Reuters. January 2018. Link: https://www.reuters.com/article/us-health-physicians-burnout/physician-burnout-takes-a-toll-on-u-s-patients-idUSKBN1F621U
- Wible, Pamela L. “Doctors and Depression: Suffering in Silence.” Medscape. May, 2017. Link: https://www.medscape.com/viewarticle/879379

–Constantine E. Kanakis MSc, MLS (ASCP)CM graduated from Loyola University Chicago with a BS in Molecular Biology and Bioethics and then Rush University with an MS in Medical Laboratory Science. He is currently a medical student at the American University of the Caribbean and actively involved with local public health.
Hematopathology Case Study: A 69-Year-Old Man Presenting with Marked Thrombocytopenia One Year after Bone Marrow Transplantation
Case history
The patient is a 69-year-old man with a history of high-risk MDS (MDS-MLD-RS) diagnosed 1 year prior to his current visit. He was successfully treated with chemotherapy and bone marrow transplantation. For the next year, several marrow examinations were normal and chimerism analysis revealed >98% donor cells. Currently, he presents with vague symptoms and a CBC demonstrates marked thrombocytopenia of 4K/μL. The low platelet count is initially thought to be related to GVHD; however, a bone marrow examination is performed to assess the status of his disease.

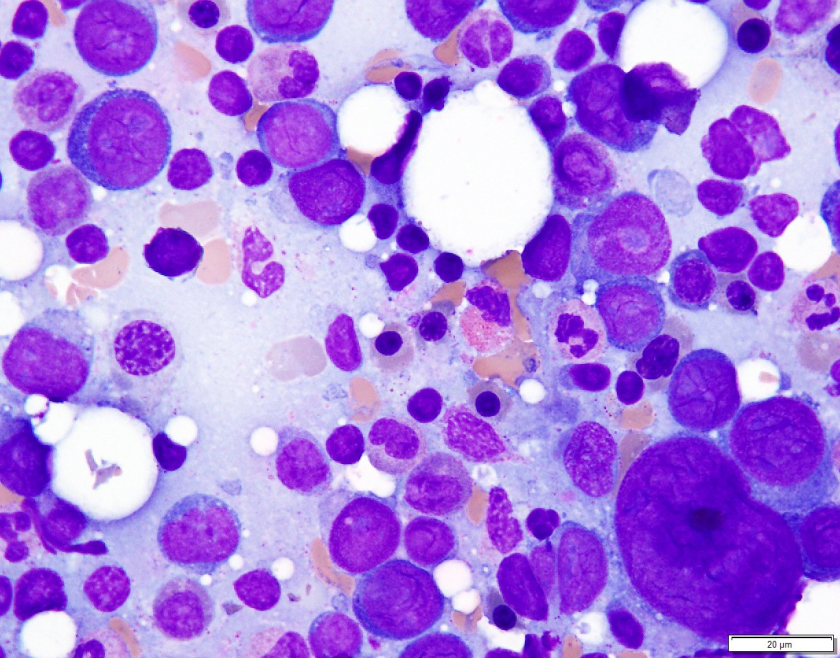

Microscopic Description
Examination of the bone marrow reveals a markedly hypercellular marrow for age with a proliferation of abnormal erythroid cells comprised of sheets of immature and maturing red cell precursors with basophilic cytoplasm. There is a marked increase in larger cells with deeply basophilic cytoplasm, prominent nucleoli, dispersed chromatin, perinuclear hoffs, and a high nuclear to cytoplasmic ratio consistent with pronormoblasts. These pronormoblasts comprised 31% of a 500-cell cell count. Additionally, the background marrow revealed a total of 81% erythroid precursors with marked morphologic atypia and dyspoiesis. Significant dysmegakaryopoiesis is noted. There is no significant increase in myeloid blasts.
Immunophenotyping
Immunohistochemical staining for E-cadherin, CD61 and CD34 is performed. These stains confirm no increase in CD34 positive blasts. CD61 highlights numerous dyspoietic megakaryocytes with widely separated nuclear lobes. E-cadherin staining is impressive, with over 80% of marrow cellularity shown to be comprised of E-cadherin positive erythroid cells.
Diagnosis
The patient’s history of MDS with current dyspoiesis, presence of >80% immature erythroid precursors with >30% proerythroblasts is diagnostic of Acute Myeloid Leukemia, NOS (Pure Erythroid Leukemia) per 2017 revision of the World Health Organization classification of myeloid neoplasms.
While successive chimerism reports thus far had shown >98% donor cells, the chimerism associated with this marrow biopsy reveals a decrease in the percentage of donor cells to 44% confirming the relapsed nature of his myeloid malignancy.
Discussion
Di Guglielmo syndrome, known as M6 leukemia in the FAB classification, was named after Giovanni Di Guglielmo, an Italian hematologist who first characterized the disease in 1917. After a few iterations in different classification schemes, the 2008 WHO Classification characterized two types of ‘erythroleukemia’ the erythroid/myeloid type and the pure erythroid leukemia. The former category of erythroid/myeloid type was removed in the 2017 update of the WHO classification with cases meeting criteria for that diagnosis now falling under the category of MDS. ‘Pure Erythroid Leukemia’ remains, and comes under the AML, NOS category, requiring >80% erythroid progenitors with > 30% proerythroblasts.
An extremely rare leukemia, PEL usually occurs as a progression of previous MDS and very uncommonly as de novo disease. Morphologically, PEL reveals proerythroblasts with deeply basophilic, agranular cytoplasm which is usually vacuolated. Occasionally, smaller ‘blasts’ with scant cytoplasm may resemble lymphoblasts. PEL is an exception to the rule of needing 20% ‘myeloid blasts’ to make an acute leukemia, since often the true myeloblast count is low.
In trephine core biopsies erythroid progenitors may take up an intra sinusoidal growth pattern with a sheet-like arrangement and typically reveal some element of background dysmegakaryocytopoiesis. When PEL lacks specific erythroid differentiation, it may be difficult to differentiate from other types of AML such as Acute Megakaryoblastic Leukemia. Park and colleagues recently categorized some under reported morphologic features of PEL and recurrent cytogenetic abnormalities associated with this disease. These findings included (but were not limited to) a broad morphologic spectrum of erythroblast morphology from undifferentiated blasts to proerythroblasts. They reported bone marrow tumour necrosis in trephine biopsies in over 70% of their cases. Of the cases wherein karyotyping was available, there was a highly complex and monosomal karyotype noted involving the TP53 gene locus.
PEL is associated with an aggressive course with a median survival of 3 months.
References
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood. 2016 Jan 1:blood-2016.
- Wang W, Wang SA, Jeffrey Medeiros L, Khoury JD. Pure erythroid leukemia. American journal of hematology. 2017 Mar 1;92(3):292-6.
- Park DC, Ozkaya N, Lovitch SB. Acute leukaemia with a pure erythroid phenotype: under-recognized morphological and cytogenetic signatures associated universally with primary refractory disease and a dismal clinical outcome. Histopathology. 2017 Aug;71(2):316-321. doi: 10.1111/his.13207. Epub 2017 May 5.

-Michael Moravek, MD is a 2nd year anatomic and clinical pathology resident at Loyola University Medical Center. Follow Dr. Moravek on twitter @MoravekMD.

-Kamran M. Mirza, MD PhD is an Assistant Professor of Pathology and Medical Director of Molecular Pathology at Loyola University Medical Center. He was a top 5 honoree in ASCP’s Forty Under 40 2017. Follow Dr. Mirza on twitter @kmirza.
The “C” in HCV Stands for “Curable”
Hi everyone! It has felt so good to find myself back in the throes of hospital life. My time in the classroom during the first half of medical school was great—but this new chapter is what makes medical school very worth it. As with any new hospital, orientation was pretty run-of-the-mill: administrative paperwork, employee/student health clearance, and yet another Mantoux PPD (despite having a current QuantiFERON—lab family, you get me).
However, after all the introductory logistics, I finally reported to my first rotation. It is an elective clerkship in primary care focused primarily on patients with HIV and/or Hepatitis. My familiarity with hospital life made the transition back easy enough as I made my way to the nurses’ station looking for my attending. Being forwarded in the direction I had to go, I knocked on the door and started to introduce myself—but was abruptly interrupted. There were already two fellow student colleagues in that room with my attending and a patient. I was enthusiastically included in the process right away, and it has been non-stop since then. I am told this is a “different” rotation where I’m going to feel lucky to have so much hands-on experience, and so far, I agree. While I reminisce on these past few weeks, it’s not a specific patient or case that has stuck with me, but an overall theme I’ve noticed in this rotation. With heavy utilization of the right test at the right time (I’m sure we’re all familiar with ASCP’s Choosing Wisely campaign) and proper interpretations of lab data, patients’ chronic illnesses are being managed well and even cured.
Essentially, pharmaceuticals have been advancing so well in the last 5-10 years that treatment regimens for chronic diseases like HIV and HCV are now being actively controlled and cured, respectively. Why does this pique my interest enough to share it with all of you? As I try my best each month to provide you a window into the life of a medical lab scientist/medical student, I do so while focusing on the lab details that seem to be present in every aspect of my journey. The cures and treatments I’m currently working with are tied to lab tests like CD4 counts, viral loads, liver and kidney function tests, and many other routine values. Diagnostic criteria for different patients’ stages of hepatic damage are classified using a Child-Pugh (CTP) score from clinical information such as ascites and encephalopathy along with lab data like INR, bilirubin, and albumin. Patients with chronic conditions come back for follow up week in and week out for lab tests that let us as care providers adjust therapy accordingly. The clinic I currently rotate in provides its patients with the most up to date treatment protocols based on current literature. For example, The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) regularly publish their recommendations for patients with Hepatitis C. It’s heavy reading, and anyone who goes through literature on standards of care knows it’s dense, so I’ll leave the link to the most recent guidelines on HCV testing, management, and treatment here (https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_g.pdf). Actively and accurately incorporating these treatment protocols into the patient care algorithms works and demonstrates great utilization of lab driven data with new available therapies.

As a baseline it is critical to understand that patients with positive HCV antibodies will always test positive; once exposed at any point patients will remain positive. While 20% of patients can clear the infection on their own, the remaining majority develop a chronic HCV infection. There is no vaccine for HCV currently; however, there is potential to cure patients—assuming the lab values are interpreted correctly. So, we’ve established that positive HCV antibodies don’t necessarily provide diagnostic data, so the next logical step is to examine a patient’s HCV viral load. Since 2015, the New York State Department of Health established a mandate and protocol for reflex testing HCV Ab positive patients with HCV RNA viral loads. Read the public letter here (https://www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/docs/reflex_testing_letter.pdf). While it makes logical sense, it’s still taking some time to get off the ground as I have seen patient records of different clinics’ providers ordering repeat HCV Ab testing for in-house confirmation—not the best use of resources or lab data. A clear example here of Choosing Wisely for the appropriate lab test. However, so long as HCV viral load stays undetectable by a validated testing method, patients with chronic HCV are promoted to a status of “cured HCV” and need no further testing or follow-up unless new clinical reasons appear to add testing as needed.

Protocols for treatment are based on things like genotype, cirrhosis, and naïve vs. previously failed treatment; treatment schedules last from 8 weeks up to 24 weeks. So, what does a patient’s first visit for HCV treatment therapy look like? Right away (assuming a positive HCV Ab has been obtained) a Hepatitis C RNA viral load is ordered, along with genotype (older treatments are dependent on genotype due to potential for resistance, while newer treatments are pangenotypic), hepatic fibrosis scans (because cirrhosis status determines length of treatment), PT/INR, CBC, CMP, HIV, RPR/CG and other STI screening, and urine drug testing. New generation therapies allow us to proceed despite any comorbid conditions, while maintaining upwards of 95% or greater cure rates. Coinfected patients with HIV or otherwise compromised immune systems are no longer contraindicated to receive HCV treatment. The only significant contraindication in the standards of care currently is that patients not be terminal (i.e. they must have a general prognosis of greater than 6 months).
Being able to watch these treatment protocols in action is great, but one patient in particular will stay with me beyond this clerkship. We received lab results back for a male in his 60s. It was his final HCV viral load based on his treatment schedule. His chart had a box at the end of his schedule labeled “test for cure” and it had remained non-detectable the whole time through treatment. The staff at this clinic does painstaking follow-up with their patients via telephone with impressive results in patient adherence and treatment success. My task one day was to call this patient and inform him that, unless he needed any medical treatment outside of his annual physical, he no longer needed to come in for therapy or testing—his Hep C was cured. He was extremely delighted to hear this news, and I was happy to give it to him. He had been on therapy for less than a few months but had lived with HCV for years. It was an excellent experience! And even more excellent—being part of the connection between lab tests, clinics, and patients. When I started I was just excited to wear that white coat and go visit the hospital’s lab, but I was pleasantly surprised to see the impact on patients’ treatments. Especially considering using the right test at the right time, and truly making a visible difference with excellent data.
See you next time!

Post script: listen to a new podcast my colleagues and I are in where we discuss clinical stories and pearls of wisdom through medical school. As they relate to my posts here on Lablogatory I’ll include a link—this post will focus more in depth on what I presented here regarding HCV cures and lab data.

–Constantine E. Kanakis MSc, MLS (ASCP)CM graduated from Loyola University Chicago with a BS in Molecular Biology and Bioethics and then Rush University with an MS in Medical Laboratory Science. He is currently a medical student at the American University of the Caribbean and actively involved with local public health.
Hematopathology Case Study: A 42 Year Old Female with Right Breast Mass
Case History
A 42-year-old female presented with a right breast mass at an outside hospital that was concerning for carcinoma. A core needle biopsy was performed of right breast mass and the case was sent for expert consultation.
Diagnosis


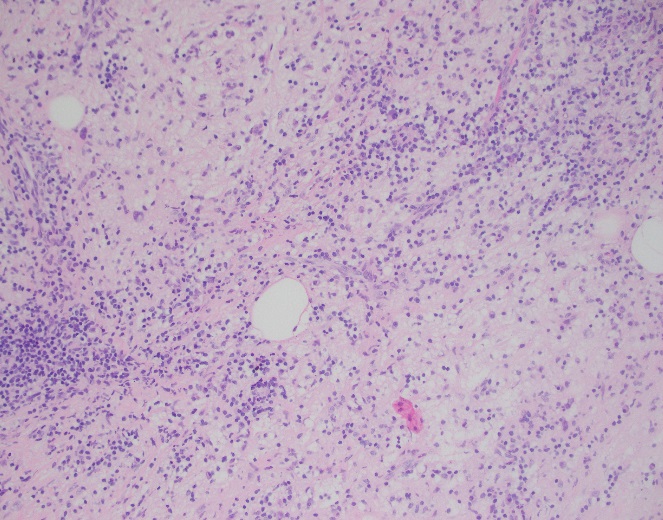
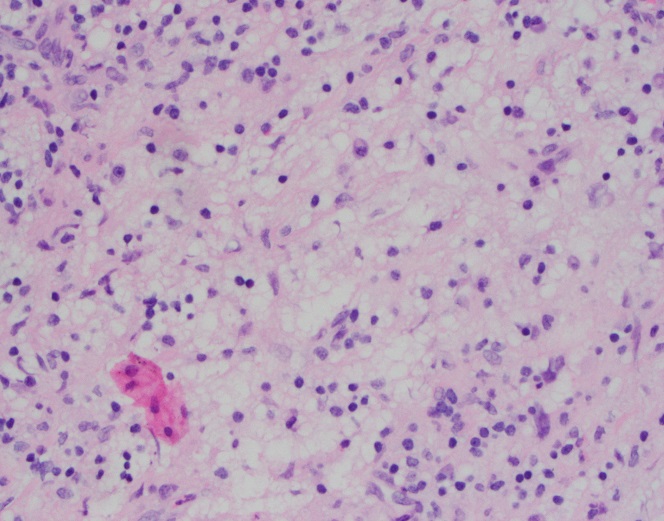
Sections of core needle biopsy material are composed primarily of adipose tissue shows a dense lymphohistiocytic infiltrate with histiocytes being the dominant cell type. Admixed plasma cells are present within the infiltrate. The histiocytes have abundant granular cytoplasm with irregular nuclear contours and some nuclei containing inconspicuous nucleoli. Frequent lymphocytic emperipolesis is identified. Immunohistochemistry performed at the outside facility show positivity for S100 and CD163 within the histiocytes, further highlighting the lymphocytic emperipolesis. Cytokeratin immunostains are negative.
Overall, the morphologic and immunophenotypic findings are consistent with a diagnosis of extranodal sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease).
Discussion
Sinus histiocytosis with massive lymphadenopathy (SHML) was first described by Rosai and Dorfman in 1969, however, similar findings may be present in extranodal sites thus earning the designation of Rosai-Dorfman disease (RDD). Although primarily present in lymph nodes, RDD may involve extranodal sites with sinuses and skin being the most frequently affected tissue types. Clinically, RDD often maintains a benign and self-limited course but may undergo exacerbations and recur, requiring surgical management. On histologic examination, RDD involves a rich inflammatory infiltrate with histiocytes, plasma cells, and lymphocytes. The histiocytes usually display a unique phenotype in which lymphocytes are phagocytosed, a process termed emperipolesis. By immunohistochemistry, these histiocytes are positive for S-100 and histiocytic markers (CD68 and CD163) and are negative for CD1a1.
The largest cohort studied involved 423 cases with 182 having extranodal manifestations2. Chest involvement was first reported by Govender et al. in 1997 in a 34-year-old female3. Overall, RDD is considered rare with a slight male predilection and young African-Americans being the most commonly affected. Sites involved ranging from most common to least common include lymph nodes, skin, upper respiratory tract, and bone4.
Extranodal sinus histiocytosis with massive lymphadenopathy, also known as Rosai-Dorfman disease, is a rare pathologic entity that histologically shows a dense lymphohistiocytic infiltrate and emperipolesis, a hallmark of the disease. Although lymph nodes are the most common site of involvement, extranodal sites may be affected and RDD should remain in the differential for lesions that contain abundant histiocytes, plasma cells, and lymphocytes as well as the classic feature of emperipolesis.
References
- Komaragiri et al.: Extranodal Rosai–Dorfman disease: a rare soft tissue neoplasm masquerading as a sarcoma. World Journal of Surgical Oncology 2013 11:63.
- Penna Costa AL, Oliveira e Silva N, Motta MP, Athanazio RA, Athanazio DA, Athanazio PRF: Soft tissue Rosai–Dorfman disease of the posterior J Bras Pneumol 2009, 35:717–720.
- Govender D, Chetty R: Inflammatory pseudotumour and Rosai–Dorfman disease of soft tissue: a histological continuum? J Clin Pathol 1997, 50:79–
- Montgomery EA, Meis JM: Rosai–Dorfman disease of soft tissue. Am J Surg Pathol 1992, 16:122–129.

-Phillip Michaels, MD is a board certified anatomic and clinical pathologist who is a current hematopathology fellow at Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA. His research interests include molecular profiling of diffuse large B-cell lymphoma as well as pathology resident education, especially in hematopathology and molecular genetic pathology.
When Rapid Blood Culture Identification Results Don’t Correlate, Part 1: Clinical Correlation Needed
More and more laboratories perform rapid (i.e., multiplex PCR) blood culture identification. For the most part, it has been a wonderful addition to the laboratory workflow, not to mention the added benefits of provider satisfaction and improved patient care. Because the PCR only provides the organism identification (sometimes only to the family-level, i.e.; Enterobacteriaceae), laboratories must continue to culture the positive blood for definitive identification and/or antimicrobial susceptibility results. So what do you do when the results don’t correlate?
The Issue
From time to time, the PCR result is not going to correlate with the direct Gram stain or with the culture results. Although this is an issue one would fully anticipate, what do you do when this happens? Do you take some sort of action to arbitrate? Do you report the results as is?
First of all, the PCR assays do not detect all organisms. They only detect the most common bloodstream pathogens. Therefore, one should fully expect to observe cases in which the Gram stain would be positive, but the PCR results would be negative (scenario 1). This is not a surprise.
Additionally, one should also assume that the PCR will occasionally detect organisms that were present at the lower limit of detection of the Gram stain. An example of this would be that the Gram stain is positive for one morphology (i.e.; Gram-positive cocci), but the PCR is positive for two organisms (i.e.; Staphylococcus and a Proteus species). Most of these cases tend to correlate with culture. In other words, although the second organism was not originally observed in the Gram stain, it was detected via PCR and then it also subsequently grew in culture (scenario 2).
Another type of discordant result laboratories sometimes experience is when the organism detected via PCR does not grow in culture for whatever reason. Similar to scenario 2 stated above, except that the culture is also negative for the second organism (scenario 3). Perhaps the patient was treated with antibiotics and the organism is no longer viable for culture? Perhaps a sampling or processing error was to blame?
The Solution
Depending on the scenario and how much work you want to do, you can either repeat testing or try an alternative method. Take scenario 2 for example. If the PCR detects two organisms and the Gram stain is only positive for one, then review of the original Gram stain is warranted. It is possible that the Gram-negative was somehow missed. Our eyes tend to go to the darker, more obvious structures. Perhaps the Gram-negative organism was faintly stained and it was overlooked? It is also possible that the Gram-positive is present in much lower numbers and only Gram-negative organism was originally observed. If the Gram stain result remains the same after review (only one organism observed), then there is nothing much left to do except to wait for the culture. That being said, an alternative method, such as acridine orange can be utilized in this type of scenario (two different cell morphologies). Acridine orange is a fluorescent stain that improves organism detection, as it is more sensitive than the Gram stain (1, 2).
If only the Proteus is growing (and the Staphylococcus isn’t from scenario 2) and we normally subculture positive blood to blood, chocolate, and MacConkey agars, then perhaps including an additional media that inhibits Gram-negative growth would be beneficial.
Scenario 3 can be a little more difficult to solve because you can’t make a non-viable organism grow. It just is what it is. [Spoiler alert: in next month’s blog I plan to write about when you should change your thinking from true-positive to false-positive.]
Regardless of why the result is discrepant, our laboratory appends a comment to the discordant result which says, “Clinical correlation needed.” This lets the clinician know that the results are abnormal and that they must use other relevant information to make a definitive diagnosis. In addition to the comment, we also make sure the discrepancy is notified to laboratory technical leadership (i.e.; Doctoral Director, Technical Lead/Specialist). This allows us to keep track of discrepancies as they may become important to know about in the future (see next month’s blog).
The Conclusion
In terms of organism detection, nucleic assays (i.e., NAATs) can provide superior sensitivity over antigen and culture-based methods of organism detection (i.e., sensitivity = PCR > culture > Gram). From the laboratory perspective, other potential benefits of utilizing nucleic acid detection methodologies include decreased TAT, simplified workflows, and reduced hands-on time. In terms of patient care, many have noted improved outcomes due to increased sensitivity and decreased time to result.
Although advances in technology can significantly improve analytical performance, they can also add complexity to the post-analytical process. Making sense of the results can sometimes lead to confusion. It is important to know the product’s limitations and what your risk(s) is. This should already be known and included in your Individualized Quality Control Plan (IQCP). Lastly, guiding the clinician to proper result interpretation is also important to maintain valuable patient care.
References
- Mirrett, S., Lauer, B.A., Miller, G.A., Reller, L.B. 1981. Comparison of Acridine Orange, Methylene Blue, and Gram Stains for Blood Cultures. J. Clin. Microbiol. 15(4): 562-566.
- Lauer, B.A., Reller, L.B., and Mirrett, S. 1981. Comparison of Acridine Orange and Gram Stains for Detection of Microorganisms in Cerebrospinal Fluid and Other Clinical Specimens. J. Clin. Microbiol. 14(2): 201-205.

-Raquel Martinez, PhD, D(ABMM), was named an ASCP 40 Under Forty TOP FIVE honoree for 2017. She is one of two System Directors of Clinical and Molecular Microbiology at Geisinger Health System in Danville, Pennsylvania. Her research interests focus on infectious disease diagnostics, specifically rapid molecular technologies for the detection of bloodstream and respiratory virus infections, and antimicrobial resistance, with the overall goal to improve patient outcomes.
Microbiology Case Study: 6 Year Old Male with Meningitis
Case History
A 6 year old male presented to the emergency department with a concern for ventriculo-peritoneal shunt (VP) malfunction. His past medical history is significant for myelomeningocele and hydrocephalus since birth. On arrival, symptoms included high fever (102.7°F), headaches and swelling at the VP shunt catheter site in the neck. Over the past week, his mother also noted nausea, vomiting and diarrhea. CT scan of the head revealed increased size of the 3rd and lateral ventricles which was concerning for either a VP shunt malfunction or infection. Lab work showed a white count of 13.5 TH/cm2 and elevated CRP values suggestive of an infection/inflammatory process. He was taken to surgery for VP shunt removal and placement of an external ventricular drain (EVD). Intra-operatively, purulent yellow material was noted at both the proximal and distal ends of the catheter. Cerebrospinal fluid (CSF) was sent for Gram stain and bacterial culture. He was started on vancomycin and ceftriaxone.
Laboratory Identification


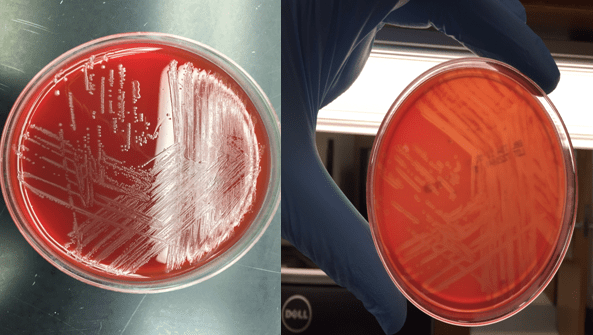
Bacterial cultures collected from a shunt tap and intra-operatively both showed short gram positive bacilli on Gram stain (Image 1&2). The organism grew on blood and chocolate agars as small, gray colonies with a narrow zone of beta-hemolysis when observed closely (Image 3) after incubation at 35°C in CO2. The isolate was positive for catalase and showed a “tumbling motility.” MALDI-TOF MS identified the isolate as Listeria monocytogenes.
Discussion
Listeria species are gram positive bacilli that grow as facultative anaerobes and do not produce endospores. The major human pathogen in the Listeria genus is L. monocytogenes and it is found in soil, stream water, sewage & vegetable matter and may colonize the gastrointestinal tract of humans and animals.
The most common mode of transmission is ingestion of contaminated foods, in particular, raw milk, soft cheeses, deli meats and ice cream. L. monocytogenes’ ability to grow at cold temperatures (4°C) permits multiplication in refrigerated foods. In a healthy adult, it causes an influenza like illness and gastroenteritis. Pregnant women are especially susceptible to disease and neonates infected in utero can develop granulomatosis infantiseptica which can lead to miscarriage, stillbirth or premature delivery. Elderly or immunocompromised can present with a febrile illness, bacteremia and meningitis (20-50% mortality).
In the microbiology laboratory, L. monocytogenes is usually identified via blood, CSF or placental bacterial cultures. It grows well on standard agars and after overnight incubation, the small, gray colonies show a narrow zone of beta hemolysis on blood agar. L. monocytogenes is positive for catalase & esculin and the CAMP test demonstrates block like accentuated hemolysis. It has characteristic tumbling motility at room temperature and an umbrella shaped motility pattern in semi-solid agar. Automated methods of identification provide reliable species level differentiation on the majority of current platforms.
Susceptibility testing should be performed on isolates from normally sterile sites. Ampicillin, penicillin, or amoxicillin are given for L. monocytogenes, and gentamicin is often added for its synergistic effect in invasive infections. Trimethoprim-sulfamethoxazole and vancomycin can be used in cases of allergy to penicillin. Cephalosporins are not effective for treatment of listeriosis.
In the case of our patient, after L. moncytogenes was identified, his antibiotic therapy was changed to ampicillin and gentamicin. Antibiotics were administered for 3 weeks before the placement of a new VP shunt. On further questioning, his mother revealed his diet consisted heavily of hot dogs and soft cheeses. She was educated on how to prevent subsequent infections prior to discharge.

-Jaspreet Kaur Oberoi, MD, is a Pathology resident at the University of Mississippi Medical Center.

-Lisa Stempak, MD, is an Assistant Professor of Pathology at the University of Mississippi Medical Center in Jackson, MS. She is certified by the American Board of Pathology in Anatomic and Clinical Pathology as well as Medical Microbiology. She is the director of the Microbiology and Serology Laboratories. Her interests include infectious disease histology, process and quality improvement and resident education.
New Year. New Skills.
I do not recall if it was an email or if I saw it on the ASCP website, but the byline caught my attention: New Year. New Skills. My mind quickly started racing. January marks a fresh beginning, the time to make new resolutions, the time to feel the excitement of new possibilities.
The Issue
We are more than halfway through the month and I have yet to identify the skill I would next like to acquire. So many questions! So much to learn, so little time! How do you choose what to focus on? Where do you start? What can you manage? Is there anyone who can help or teach you? And if you are like me, you might also ask yourself, “Why do I always pile more on my plate?” Maybe this is the year you choose to learn to say no? Nah. So what’s it going to be?
The Solution
Since our lives are all different and there are millions of possible distinct scenarios, I will share what I decided to do. First, I evaluated my work-life balance and determined if I wanted to acquire a skill that would benefit my work (career and ambition) or lifestyle (health, pleasure, leisure, family) (1). I also took into consideration how much more I could fit onto my already overflowing plate.
I decided to work on something that would help me with both work and lifestyle (because who doesn’t like to maximize their return on investment?). I chose something I do not like to do, something that scares me, something I have difficulty with, something I avoid like the plague, but most importantly it’s something that I wish I could do better; a skill that I envy: having difficult conversations.
Communication is a vital component of our lives. We all communicate, but how many of us have mastered the skill of communicating? Also, there are many aspects of communication (2). Poor communication can make or break a situation or relationship. Being able to communicate well is a great skill to possess (3). Reference two provides a long list of skills that I highly recommend you also take a look at (https://www.thebalance.com/communication-skills-list-2063737). I went down the list and individually assessed which skills I feel that I do well with and which ones I do not (2). This little exercise served as a reality check as to where I stand in regard with my aptitude to communicate. I invite you to do the same. You may be surprised at what you find!
The Importance of Good Communication
As a laboratory director, many facets of my job depend on my ability to communicate well. I must communicate with clinicians, technologists, administrators, other coworkers, vendors, students, etc. Not only do I communicate with a variety of groups of people, in a multitude of different platforms (individually, small groups and meetings, or large groups; such as national conferences), but it is also important that my written, verbal, and non-verbal communication skills are clear and easily understood.
As laboratory professionals, one very important aspect of our job is to communicate critical results. It is essential that we not only relay the data, but it is equally important for us to communicate it well so that the clinician completely understands the information so that they can properly care for the patient. Moreover, we must not forget the golden rule: garbage in, garbage out. What I mean by this is that good communication should begin in the pre-analytical phase. We want the clinician to provide the laboratory with the best possible specimen so that in turn, we can provide them with the most accurate result. So how do we ensure that we obtain the best possible specimen? We communicate.
The laboratory communicates our needs to the provider in order to properly do our job. For example, we provide detailed information on how to properly collect specimens, which container type to use, how to handle the specimen, how much (volume) specimen to submit, which temperature to submit the specimen, etc. Properly communicating these details is essential.
The Difficult Conversation
As laboratory professionals, we are just one part of a larger healthcare team. If you stop to think about it, we all have to participate in difficult conversations as part of our jobs. Doctors have to tell patients that they are going to die, laboratory professionals have to tell clinicians we lost their specimen, executive administrators have to tell downstream leadership that the budget has been cut again, managers and supervisors have to tell employees they are being written up or worse. Being able to successfully have a difficult conversation would serve us all well. As such, most institutions provide classes or webinars to help employees develop this skill.
The definition of difficult is: not easily or readily done; requiring much labor, skill, or planning to be performed successfully; hard (4). Carrying out a difficult conversation with grace is an extraordinary skill that encompasses a variety of communication attributes. Regardless of the scenario, the communicator must be clear, articulate, and courteous. However, depending on the scenario, being concise, confident, strategic, diplomatic, convincing, empathetic, motivating, open-minded, and/or quick thinking may also be useful skills to possess during a difficult conversation. Other valuable skills are conflict management, being able to explain, and/or listening.
The Conclusion
For many, the New Year marks the time to set new goals, to accept new challenges, and welcome new beginnings. Why not use this opportunity to learn a new skill? The good news is that no matter what your new skill will be, it will also benefit your health. In order to acquire a new ability, you must work to actively learn to become proficient in that ability; therefore learning a new skill will also benefit your brain function. There are many studies that demonstrate that active learning keeps the mind sharp (5). Challenging your mind improves brain function and active learning slows cognitive decline (6). If you want to be brave, then don’t only choose a skill that will be fun or helpful, but choose to learn something that also challenges you to face one of your fears. For me, I hope to learn how to master the art of having difficult conversations….successfully. In the words of Marie Curie, “Nothing in life is to be feared, it is only to be understood. Now is the time to understand more, so that we may fear less.”
Happy learning! Happy New Year!
The References
- Work-life Balance. https://en.wikipedia.org/wiki/Work–life_balance. Accessed January 16, 2018.
- The balance. List of Communication Skills for Resumes. https://www.thebalance.com/communication-skills-list-2063737. Accessed January 16, 2018.
- The balance. Communication Skills for Workplace Success. https://www.thebalance.com/communication-skills-list-2063779. Accessed January, 16, 2018.
- com. Difficult. http://www.dictionary.com/browse/difficult. Accessed January 16, 2018.
- Stenger, M. 2013. New Study Shows How Active Learning Improve Cognitive Function. https://www.opencolleges.edu.au/informed/other/new-study-highlights-activities-to-improve-cognitive-function-6008/. Accessed January 17, 2018.
- Park, D.C., Bischof, G.N. 2013. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci. 15(1): 109-119. PMC23576894. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3622463/. Accessed January 17, 2018.

-Raquel Martinez, PhD, D(ABMM), was named an ASCP 40 Under Forty TOP FIVE honoree for 2017. She is one of two System Directors of Clinical and Molecular Microbiology at Geisinger Health System in Danville, Pennsylvania. Her research interests focus on infectious disease diagnostics, specifically rapid molecular technologies for the detection of bloodstream and respiratory virus infections, and antimicrobial resistance, with the overall goal to improve patient outcomes.
Hematopathology Case Study: An 18-year-old Man with Acanthocytosis
Case History
We were asked to review the peripheral blood smear of an 18-year-old male who had presented to the emergency department with shortness of breath and abdominal distension. His past medical history was significant for numerous hospitalizations for recurrent fungal and bacterial pneumonia, pulmonary abscesses, osteomyelitis, necrotizing granulomas, and cervical lymphadenopathy requiring multiple lymphadenectomies. This history dates back to when he was 3 months old.
Blood Smear findings
The CBC demonstrated severe anemia and mild leukopenia. The peripheral blood smear showed numerous acanthocytes and poikilocytosis shown below.


Additional Clinical Findings
Abdominal ultrasonography demonstrated hepatosplenomegaly with enlarged porta-hepatis lymph nodes. Additionally, chest CT scanning demonstrated bilateral mass-like consolidations, prominent hilar lymphadenopathy, and osteolytic lesions of the vertebral bodies. A comprehensive investigation for opportunistic infections was negative. Lung and vertebral body biopsies (not pictured here) revealed poorly formed granulomas. A blood transfusion was considered; however, the patient had previously been demonstrated to express anti-Kx antibodies, which would require transfusion with exceedingly rare blood products.
Diagnosis
The preceding case history describes a patient with a contiguous gene deletion syndrome that includes chronic granulomatous disease (CGD) and the McLeod phenotype, demonstrating a fascinating disorder with important implications in hematopathology and several other disciplines of pathology.
Discussion
McLeod syndrome is a rare, X-linked disorder characterized by the deletion of the XK gene which encodes for the Xk protein. Overall, the lack of synthesis of the Xk protein leads to the lack of expression of the Kx antigen which in turn leads to a marked decrease in the quantities of Kell antigens. In this case, due to the presence of an anti-Kx antibody, the patient would require transfusion with either Kell-null or McLeod phenotype blood products. Unfortunately, only one unit of compatible blood was identified when the rare blood donor database was queried. The clinical team therefore elected for treatment with erythropoietin and iron supplementation which eventually lead to a modest increase in the patient’s hemoglobin concentration.
Acanthocytes, or spur cells, are spiculated red cells with a few projections of varying size and surface distribution that can be seen in a variety of clinical conditions including CGD with McLeod red cell phenotype. Other conditions include (but are not limited to) neuroacanthocytosis, malnutrition states, infantile pyknocytosis, (Lu) null Lutheran phenotype, hypothyroidism, myxedema, and Zieve syndrome. Acanthocytes should be distinguished from echinocytes, or burr cells, that also demonstrate multiple small projections but these are uniformly distributed on the red cell surface.
The prominent acanthocytosis seen in McLeod syndrome is thought to be due to an imbalance of the number of lipids in the inner layer relative to the outer layer. Related to this phenomenon is McLeod neuroacanthocytosis syndrome, a disorder with neurologic manifestations including movement disorders, cognitive alterations, and psychiatric symptoms. Although our patient did not exhibit these symptoms, McLeod neuroacanthocytosis syndrome is known to start in early to middle adulthood and the patient will need to be monitored for the onset of neurologic sequelae.
The McLeod phenotype is frequently associated with CGD due to the proximity of the XK gene to the CYBB gene on the X chromosome. The CYBB gene encodes for a subunit of the NADPH oxidase enzyme complex. A deficiency in NADPH oxidase activity leads to the characteristic increased susceptibility to severe bacterial and fungal infections seen in CGD. The nitroblue-tetrazolium test can be used to evaluate NADPH oxidase activity in the white blood cells and can help make a diagnosis of CGD. Histologically, CGD can show prominent necrotizing and non-necrotizing granulomas in various locations throughout the body.
Overall, treatment of CGD with McLeod red cell phenotype is supportive. There is no known cure or definitive treatment. The patient will likely continue to have infections with opportunistic organisms which will be treated on a case by case basis.
References
- Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Current opinion in immunology. 2003 Oct 31;15(5):578-84.
- Jung HH, Danek A, Walker RH, Frey BM, Gassner C. McLeod neuroacanthocytosis syndrome.
- Khodadad JK, Weinstein RS, Marsh LW, Steck TL. Shape determinants of McLeod acanthocytes. Journal of Membrane Biology. 1989 Mar 1;107(3):213-8.
- Watkins CE, Litchfield J, Song E, Jaishankar GB, Misra N, Holla N, Duffourc M, Krishnaswamy G. Chronic granulomatous disease, the McLeod phenotype and the contiguous gene deletion syndrome-a review. Clinical and Molecular Allergy. 2011 Nov 23;9(1):13.

-Michael Moravek, MD is a 2nd year anatomic and clinical pathology resident at Loyola University Medical Center. Follow Dr. Moravek on twitter @MoravekMD

-Kamran M. Mirza, MD PhD is an Assistant Professor of Pathology and Medical Director of Molecular Pathology at Loyola University Medical Center. He was a top 5 honoree in ASCP’s Forty Under 40 2017. Follow Dr. Mirza on twitter @kmirza.
