A CBC was received on a 70-year-old surgical inpatient at our facility and was run in automated mode on our Sysmex XN analyzer. On the first run, the analyzer gave flags for RBC agglutination and MCHC >37.5. These flags require evaluation of the high MCHC with investigation of a cold agglutinin, lipemia or icterus. A smear review for RBC agglutination was also indicated. The sample was incubated at 37°C for 30 minutes and the CBC was repeated. Results are shown below in Table 1.
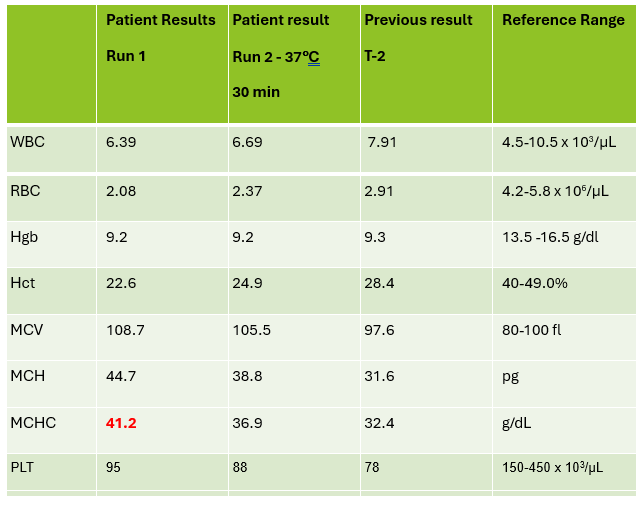
On Run 2, the MCHC is now below 37.5, so there was no operator alert to incubate further or to investigate the high MCHC. This result was validated with the comment “37°C results, possible cold agglutinin.” However, there was still an RBC agglutination flag, a high MCV, and the hemoglobin and hematocrit still don’t look great, i.e. they don’t follow ‘the rules of 3’. We know that these rules are only valid for normal samples, but if we look at the previous results from 2 days ago, the Hgb 9.3 and Hct 28.4 did follow the ‘rules of 3’ and additionally, today’s results look like the patient’s hemoglobin has not changed but the hematocrit has dropped. The RBC did go up on the second run, but it is considerably lower than 2 days ago. The indicies are also inconsistent with the previous sample. “Hmmm…What could cause this?” Instead of validating, this is what you should be asking yourself.
Cold agglutinins are known for causing pre-analytical and analytical spurious results with CBCs. With cold agglutinins, IgM antibodies bind to the RBCs after exposure to cold, causing RBC agglutination which leads to a classic pattern of false results. There is an increased MCV because the RBCs are clumped and sticking to one another, making the analyzer think these larger clumps are individual RBCs. This, in turn, will make the RBC count appear deceased because large clumps of RBCs going through the RBC aperture are counted as one RBC. The hematocrit is lowered because the volume of a clump is less than those cells individually. Hemoglobin concentration, on the other hand, is not affected by cold agglutinins, but because the hematocrit and RBC are falsely lowered, this makes the MCH and MCHC spuriously markedly increased. Cold agglutinins may be subtle, like this one, but some have extremely high MCHC and MCV, and extremely low RBC and Hct. Clues that you have a cold agglutinin are not only the high MCHC, but also flags from your analyzer such as “RBC agglutination”. The hematocrit will likely seem too low for the hemoglobin, and you may even see a hemoglobin that is higher than the hematocrit (yes it happens!) and RBC values so low as to be incompatible with life.
The first thing is to do if you get these spurious results is to compare the parameters and instrument flags and decide if the results are consistent with a cold agglutinin. Other factors that may cause a high MCHC include lipemia, icterus, low sodium, abnormal proteins, hemolyzed samples and samples from patients with hemoglobinopathies. The patterns of result with these samples will show a high MCHC, but with a normal or low MCV, and you won’t usually see an RBC agglutination flag. A sample with cold agglutinins may also appear grainy or clumpy to the naked eye. With a suspected cold agglutinin, warming the sample for 15-30 minutes will allow the RBCs to disperse and improve the results. However, these results must be reviewed, and if there are still instrument flags and/or if there is still clumping, the results may not yet be corrected or ‘correct’. Be sure to review a smear from the tube before warming and after. The first smear confirms the presence of the cold agglutinins and clumping, and the second smear should confirm the resolution of the clumping after warming.
This sample did have the characteristic grainy appearance of a cold agglutinin, and the post warming results did look a bit better, but the RBC agglutination flag was still present, and a review of the smear showed that the sample still had RBC clumping. After warming for another 30 min, the sample was quickly mixed and placed back on the analyzer. The results of this 3rd run are shown below, in Table 2.
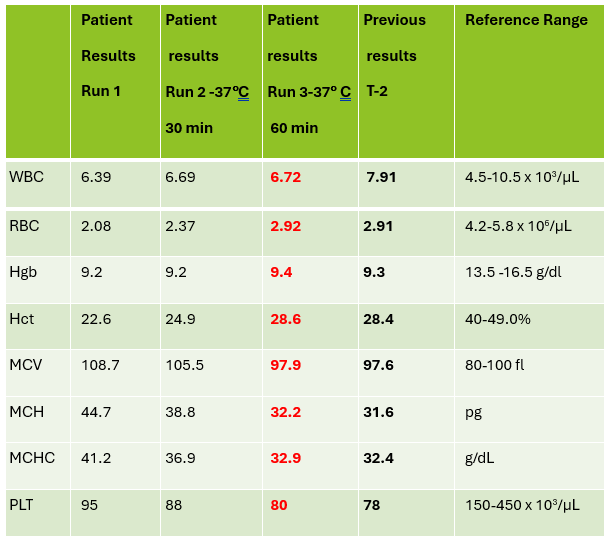
Notice that hemoglobin has not changed as it is not affected by the cold agglutinins, but after warming for 60 minutes, the RBC, hematocrit and indicies all now look consistent with the previous sample drawn 2 days ago, and a review of the smear showed no RBC agglutination. These results from the 3rd run are ready to validate.
Cold agglutinins are IgM autoantibodies that react best at 4°C but may also react at room temperature. They are generally not clinically significant and may be found in many healthy individuals. These natural cold autoantibodies occur at low titers, less than 1:64, and have no activity at higher temperatures. However, because they react at room temperature, they are notorious as a pre-analytical and analytical factor that causes spurious CBC results. They can also cause difficulties in Blood Banking during ABO/Rh typing and antibody detection.
Cold agglutinins have various clinical manifestations. Benign cold agglutinins generally do not cause hemolytic anemia and need no treatment. Most benign cold autoantibodies have anti-I specificity, are polyclonal, low titer, and do not react above 30°C. Cold agglutinins associated with Mycoplasma pneumoniae, and infectious mononucleosis are usually clinically insignificant. In cases where they do cause hemolytic anemia, the antibodies are polyclonal IgM with normal κ and λ light chains. The anemia is acute and generally spontaneously resolves in several weeks without treatment.
Though most cold agglutinins are benign and do not cause RBC destruction, when they do, they can cause hemolytic anemia that varies in severity from mild to life-threatening. This chronic cold agglutinin disease (CAD) is now known to be a form of autoimmune hemolytic anemia caused by a bone marrow lymphoproliferative disorder. Chronic CAD is a cold-autoantibody autoimmune hemolytic anemia (cAIHA) that is caused by an autoantibody produced by the clonal B cell lymphocytes. This antibody is usually monoclonal IgM with κ light chains and “I” or “i” specificity. These pathological cold agglutinins are high titer and usually react at 28°C to 32°C, and even up to 37°C. The highest temperature at which the antibodies continue to be activated is called the thermal amplitude. Because these can act at higher thermal amplitude, they may lead to CAD. In CAD the IgM autoantibodies bind to red cell antigens at 30-32°C, typically in the cooler extremities. IgM’s structure, a large immunoglobin pentamer, makes it an effective activator of the classical complement system. As the blood circulates to the central parts of the body, the RBCs warm up and the IgM antibodies dissociate from the RBC membranes, but the complement activation will continue, leading to RBC hemolysis and a cAIHA.
Chronic CAD occurs most often in adults over 50, is more common in women, and produces anemia with varying severity. Patients may be seasonally affected. In the winter, the temperature of blood may fall below 30°C in the extremities, activating the cold agglutinins. Patients may experience acrocyanosis of the hands, feet, ears, and nose with exposure to cold. They may also experience other cold related symptoms such as numbness and Raynaud’s. Patients with chronic CAD and mild anemia are therefore monitored with a ‘wait and see’ plan and advised to avoid cold temperatures. In patients with more severe anemia, it is found that targeting the underlying lymphoproliferative disorder provides the best treatment. Rituximab has been used to achieve partial remission. Therapeutic plasma exchange is also used in severe cases to rapidly remove cold agglutinins.
I have been thinking about cold agglutinins recently because of the number I have seen come into our lab this winter. As I am writing this, watching the temperature outside drop in anticipation of more snow coming in tonight, cold agglutinins came to my mind again. I used to live in the cold northern Northeast, but after moving further south, we see fewer cold agglutinins in hematology than I used to see. This winter we have had some cold spells, and, interestingly, I’ve seen more cold agglutinins. That led me to ask myself if cold agglutinin disease is really more common when patients are exposed to cold temperatures. I remember learning myself, and telling my students that that the treatment for mild to moderate CAD was to advise patients to move to someplace warmer. It has been assumed for many years that CAD worsens in colder climates or seasons. Interestingly, there have been a number of studies done since the 1950’s that examined the relationship of cold temperatures and CAD. The studies used hemoglobin, bilirubin and LDH for monitoring. Early case reports had findings that supported the theory of more anemia and higher LDH in the winter. (Dacie, Lyckholm)One recent article in 2022 found a 4-fold difference in the incidence of CAD between cold (Norway) and warm (Italy) climates. (Berentsen). However, atthe same time another study found that there was no statistically significant seasonal variation in hemoglobin, but that LDH levels were higher in winter. It concluded that these conditions should be monitored through all seasons because of the risk of hemolysis and thrombotic episodes. It was also demonstrated that though there may not be obvious statistical difference in CAD between cold and warm months, that there is a large variability of disease severity across patients and even with an individual patient. (Roth).
In conclusion, when working in any department of the laboratory, quality results are important. Results on the patient chart that vary considerably from day to day because sometimes a cold agglutinin has been effectively resolved in lab testing and other days results after 15-30 minutes of warming are just reported without a good review of the smear and the parameters, are confusing, and could affect patient care. If one tech reports the results after 30 min incubation with values that are still spurious, and the next tech resolves the agglutination with further warming, the lab will be reporting out inconsistent results. A patient who actually has stable CBC results may have deltas and what appear to be erratic results. Cold agglutinins do take time to resolve, but with over 80% of samples autoverifying with the use of auto verification, we have time to work on these problem samples. If something doesn’t look or feel right about a sample, look at all the parameters, check the instrument flags and operator alerts, check the previous results and investigate any changes. It is important to review results carefully, because we want to report out the best results possible.
References
S Berentsen, W Barcellini, S D’Sa, U Randen, THA Tvedt, B Fattizzo, E Haukås, M Kell…
Blood, The Journal of the American Society of Hematology, 2020•ashpublications.org
Climent F, Cid J, Sureda A. Cold Agglutinin Disease: A Distinct Clonal B-Cell Lymphoproliferative Disorder of the Bone Marrow. Hemato. 2022; 3(1):163-173. https://doi.org/10.3390/hemato3010014
Nikousefat Z, Javdani M, Hashemnia M, Haratyan A, Jalili A. Cold Agglutinin Disease; A Laboratory Challenge. Iran Red Crescent Med J. 2015 Oct 17;17(10):e18954. doi: 10.5812/ircmj.18954. PMID: 26566452; PMCID: PMC4636857.
Patriquin, C.J. and Pavenski, K. (2022), O, wind, if winter comes … will symptoms be far behind?. Transfusion, 62: 2-10. https://doi.org/10.1111/trf.16765
Rodak, Bernadette F., et al. Hematology: Clinical Principles and Applications. 5th ed. St. Louis, Mo., Elsevier Saunders, 2016
Röth A, Fryzek J, Jiang X, Reichert H, Patel P, Su J, et al. Complement-mediated hemolysis persists year round in patients with cold agglutinin disease. Transfusion. 2022; 62: 51–59. https://doi.org/10.1111/trf.16745

-Becky Socha, MS, MLS(ASCP)CMBBCM graduated from Merrimack College in N. Andover, Massachusetts with a BS in Medical Technology and completed her MS in Clinical Laboratory Sciences at the University of Massachusetts, Lowell. She has worked as a Medical Technologist for over 40 years and has taught as an adjunct faculty member at Merrimack College, UMass Lowell and Stevenson University for over 20 years. She has worked in all areas of the clinical laboratory, but has a special interest in Hematology and Blood Banking. She currently works at Mercy Medical Center in Baltimore, Md. When she’s not busy being a mad scientist, she can be found outside riding her bicycle.