Hello again everybody, and welcome back! Last month, I was flattered by a double feature with my post about giving a TEDx talk and Dr. Razzano interviewing me for her global health series. This month, I’d like to address a topic that’s been literally everywhere lately and is just as hard to ignore as…well, second-hand smoke. So, fasten your seatbelts, ensure your seats and tray tables are in the upright position, make sure your biases are stowed in the seat before you, and (of course) please note the no smoking sign as we take off on the topic of vaping!

The Smoking Gun
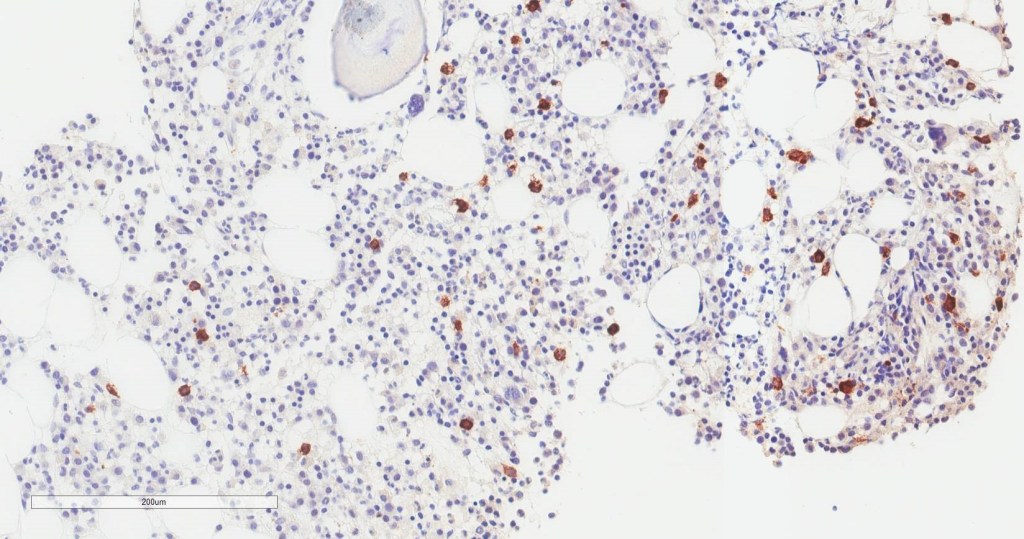
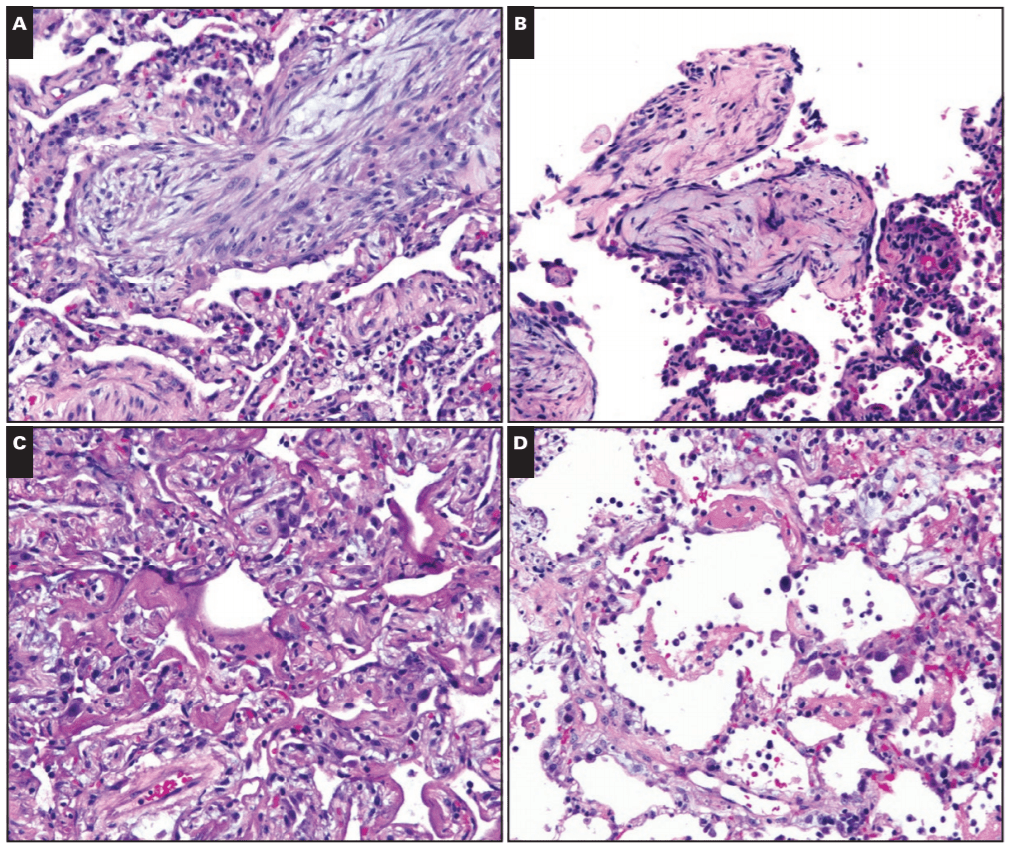
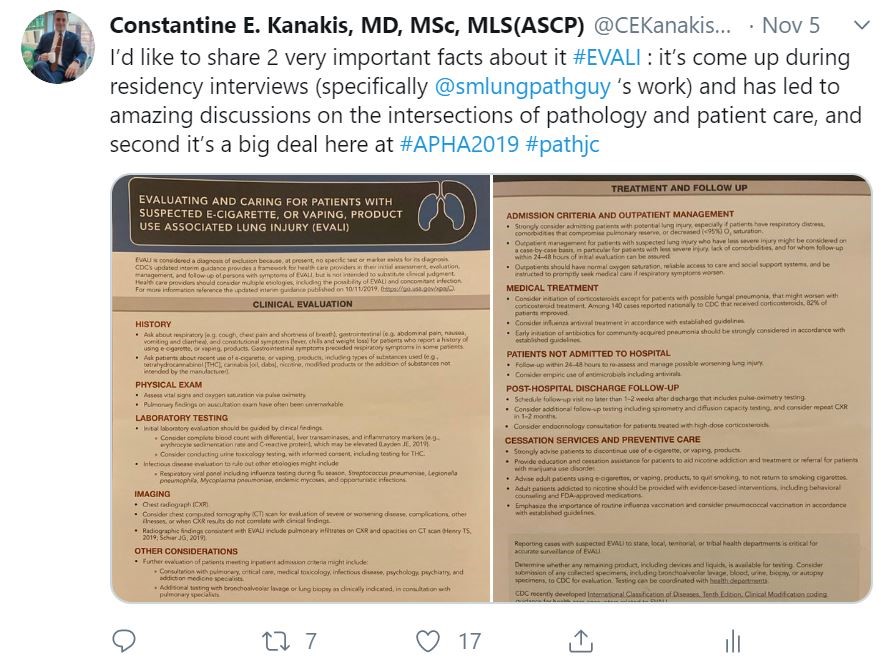
You may have noted that in the past few weeks or months the topic of vaping has been a mainstay of nighttime news stories and front-page print articles. That’s because there’s a lot happening, and from a lot of different angles. It can be messy and confusing, especially because there’s a scientific and non-scientific debate: availability, marketing, health risk, research, and more—all happening at once. I’m going to talk a little bit about all of this, but mostly we’ll look at the medical aspect of vaping as some fantastic publications are making their way into medical journals, including our very own American Journal of Clinical Pathology (AJCP). Recently, friend, colleague, and fellow member of the ASCP Social Media Team and pulmonary pathologist at the Cleveland Clinic, Dr. Sanjay Mukhopadhyay (@smlungpathguy on Twitter) published a noteworthy article with AJCP demonstrating the histopathologic findings of vaping associated lung injury. In essence, vaping causes acute lung injury which is recognized in tissue, supporting the case that both further studies are mandated for health and safety and that vaping should currently be considered a potential critical health risk.

In this paper, Dr. Mukhopadhyay, et al, tried to capture the direct tissue-related effects of vaping. EVALI, Electronic-Cigarette or Vaping use Associated Lung Injury, has received quite a bit of spotlight in the media as I mentioned. Case series featured in the New England Journal of Medicine (NEJM) highlighted patients in the Midwest with EVALI-type pulmonary disease, but the number of publications on the topic is currently scarce—let alone ones that demonstrate the actual pathophysiology in-process in those affected patients. In the AJCP paper, lung biopsies from a small number of male patients who havdrespiratory illness and concurrent histories of vaping were examined. With all other pulmonary pathology worked up and negative, their biopsies showed various patterns of acute lung injury. The NEJM cases were also worked up and found to be negative for the differentials of pulmonary disease whether infectious, inflammatory, or otherwise; adding credence to a developing body of research supporting the connection between vaping and EVALI.

Where There’s Smoke, There’s…a Lot of Stuff, Actually
There are a ton of stories in the lay-press about vaping-related illnesses. The surveillance data from those NEJM case series and the CDC show a median age of 19 with an overwhelming 94% being hospitalized and roughly two-thirds of those requiring ICU intervention and one-third having to be placed on mechanical ventilation. Of note, 11% of these patients claimed that they vaped pure nicotine product, while 89% smoked cannabinoids/THC in their vape products. Most of them presented to medical care with oxygen saturations <89% on room air (normal O2 sats are variable by patient, but they should be above 95% in ideally healthy individuals). This is neither an endorsement or comment on the medical uses of cannabinoids or a statement on their health effects. Instead, it should be worth mentioning that not only are electronic-cigarette products a new way of smoking higher concentrations of tobacco-obtained or synthetic nicotine but also other products, which have very little data with regard to their associated health risks.

Put This in Your Pipe and (please don’t) Smoke It
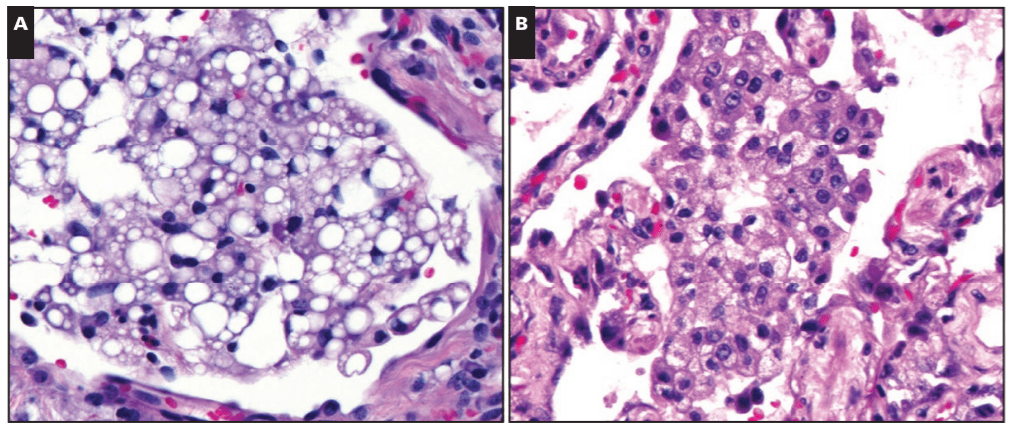
Okay, I mentioned cannabinoids. Now that I have your attention, I want to walk you through a unique piece of the EVALI discussion you may have seen in the media: the implication of Vitamin-E substances as a potential culprit for these lung-related injuries. The New York Times recently published a piece that cites the CDC’s consideration of Vit-E Acetate as a “a very strong culprit.” Think about it this way: the aerosol generated by vaping devices can reach very high temperatures (higher than traditional cigarettes), if a substance is inhaled at this temperature, and contains lipid-soluble-contents like Vitamin-E acetate, you’re breathing in a grease fire! Here’s an oversimplification: some studies of vaping came up with a theory that a grease fire would cause injury in the lungs similar to a pattern caused by inadvertent inhalation of mineral oil into the lungs known as “exogenous lipoid pneumonia”. However, when expert lung pathologists including Dr. Mukhopadhyay looked at lung biopsies from EVALI patients, they didn’t find even a single case of exogenous lipoid pneumonia. What does this mean? Not much at this point. It’s certainly possible that vitamin E acetate causes lung damage but not in the way mineral oil does. As the CDC materials state, this is early days if it is indeed a health epidemic (it probably is though, please stop vaping). More research is needed, as always, but you can read the NYT article and CDC primer article here.
Fired Up, Ready to Go and Sending Smoke Signals
So, imagine you’re a vaper. Imagine you started because it helped you quit traditional cigarettes. That’s fantastic, good for you. You’re on the road to smoking cessation and better health! But perhaps the vaping-associated lung injury cases has made you a little defensive. Trust me I learned the hard way as I joined in the discussion earlier this month on a live-tweet pathology journal club on the AJCP article featured here. They happen under the hashtag #PathJC and lots of folks jump into the discussion from different places, institutions, time zones, and across disciplines—but its not just a bunch of pathologists analyzing an article in an academic bubble. Twitter is a public forum and that brings with it public scrutiny and commentary. As such, there were lots of lay people participating in the discussion and many individuals who held a positive opinion of electronic cigarettes. So not only did we have a very comprehensive discussion in the merits and shortcomings of published literature on the topic of EVALI, we also had to field questions and engage in non-jargon conversations with concerned (and sometimes passionate) members of the non-scientific community. Suffice it to say, it’s a tricky tightrope to walk when you’re trying to balance your anti-smoking public health crusade with some good old-fashioned medical education challenged with a sprinkle of vitriol on the most open of forums, the internet. But that’s okay! I strongly think, that in the future of medical practice, those of us in any discipline (but especially pathology and lab medicine) should lead the charge as champions of truth to connect our revered medical data to people in real terms—basically translate translational medicine.

Once the Smoke Settles
Basically, everything’s going to be okay. There’s always a crisis or an epidemic happening that we have to address with limited data, developing knowledge, and some cohort of representative push back. That’s the nature of public health. But I’ll pull straight from the authors’ conclusion in the AJCP paper and remind you that not only is this just one, single study with very small number of cases to measure clinical outcomes, but further study is needed to support what is just beginning to be a correlation between vaping and lung injury.
TL;DR – it might seem obvious to some that hot smoke burns your lungs, but we’ve got to prove it and take steps to protect our patients everywhere.
And the good news is there are lots of us working on this. Scientists, public health officials, researchers, reporters, medical professionals, and especially pathologists are here collecting data and adding knowledge to that growing body of evidence to address this …hot topic.

Breathe Easy
What’s past this smokescreen challenge? The same thing as always: hard work, collaboration, innovation, and paradigm shifting. If you’ve read my previous posts, you know I like to wax a bit about the future of medicine and the humanity behind our profession. Taking everything into consideration with this newest and hottest of public health concerns, our role as diagnosticians and translational representatives is as important as ever. And, if we want to ensure the recognized contributions of pathology in the wider field of medicine (and health-at-large) we should work with our colleagues in and out of the medical profession to demystify this kind of research, cleanly communicate health data to the public, and push the boundaries of personalized health and improved patient outcomes. But beware: when you address big topics like smoking, vaping, EVALI, and THC use, it can be easy to get too hot, and even burn out.
Thanks again, see you next time, and hope you had a Happy Thanksgiving!
(This is absolutely stolen from @iHeartHisto on Twitter, but enjoy a slice of pump-skin pie!)


–Constantine E. Kanakis MD, MSc, MLS (ASCP)CM completed his BS at Loyola University Chicago and his MS at Rush University. He writes about experiences through medical school through the lens of a medical lab scientist with interests in hematopathology, molecular, bioethics, transfusion medicine, and graphic medicine. He is currently a 2020 AP/CP Residency Applicant and actively involved in public health and education, advocating for visibility and advancement of pathology and lab medicine. Follow him on Twitter @CEKanakisMD