Hello again everyone!
After a lot of positive responses and sharing on social media, my article last month got lots of people talking about annual meetings and how great they are for networking, learning, and advancing our profession. Not too long after the ASCP Annual Meeting in Phoenix, I was back in my Manhattan apartment working on my speech and graphics for a real life TEDx session hosted at my medical school.
Let’s pause here: if you either haven’t heard of the TED/TEDx brand or if you binge watch their 18 minute videos and want more links to watch now, now, now!
TED is a non-profit organization whose mission is to share “ideas worth spreading.” They’re about 35 years old and based in NYC stateside, and Vancouver in Canada. Basically, over the last few decades they hold conferences at those flagship sites called “TED talks” where selected speakers present on a myriad of topics. TEDx conferences are officially licensed but off-site events which operate under TED protocol and guidelines. There have even been spin-off conferences like TED MED, which focus solely on healthcare.

Some of the students at AUC School of Medicine, organized such a conference with official TED licensing and recruited me to join their list of speakers to deliver talks on their chosen theme: resilience. Officially called TEDxAUCMed, this conference included community members, students, artists, activists, and more discussing the human capacity for resilience in ways not commonly discussed. “Weathering the Storm” was the official event title, as the school located in the island nation of St. Maarten displays daily resilience especially since being hit by Hurricane Irma in 2016. Among their list of incredible speakers, I was humbled to be included! I titled my talk “Unrecognizable Medicine” and wanted to deliver a talk to students, clinicians, and those of us in medicine witnessing first-hand a tidal wave of new technologies and paradigms that redefine the way we discuss health. Oh, and since I’m a huge fan of #GraphicMedicine more and more each day, I hit that hashtag hard and decided to illustrate my whole talk!

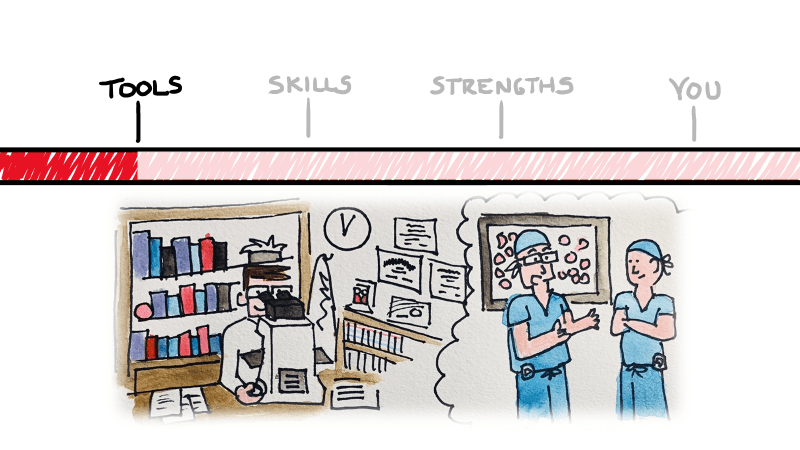
So what did I talk about, exactly…and what’s the big deal? I’m not going to re-hash my presentation for you in text—that’d be boring, and I’m obviously going to put a link at the bottom for you to watch it yourself. I got you, lab fam! But essentially, what I set up was a three-tiered template to assess and navigate that tidal wave of tech. Tools, skills, and strengths—three things inherent to the practice of medicine in any specialty.

There are untapped topics in medicine which are looming over the horizon. As medicine continues to evolve and change, the problems we face and the needs we must meet will become moving targets. New specialties will emerge, and new technologies will replace centuries old tools we cling to today. A shift in thinking is both proactive and healthy in a profession that mandates our commitment to preserving health and quality of life. I have spent years battling stereotypes in medicine and hope to challenge the fabric that places individuals in professional or academic boxes. Fresh first-years at some schools are already using point-of-care ultrasounds (POCUSes) instead of stethoscopes—which student sounds like they have better info on morning rounds, a student who maybe kinda-sorta heard some non-descript murmur, or a mini-pocket echocardiogram with an ejection fraction of 45%? Stereotypes have too long shaped the way students choose specialties, equating some areas to colloquial high school cliques! No offense to orthopedics or dermatology. Troponins used to be something you could hang your white coat on, but not anymore. What do you do with a new 5th generation Trop of 39 with a delta of 18? ACS or acute MI? Cancer therapy is exploding with personalized treatments being added every day! Any student right now would impress their heme/onc attending on rounds if they suggested PDL-1 and other immunotherapy testing for patients with newly diagnosed lung cancers. *Deep breath*
Ok. My point is, tomorrow’s medicine is going to have a lot of different therapies, tools, and even vocabulary that schools may never catch up with. How do you prepare for this explosion of knowledge? You look to yourself to take an inventory of your strengths and use those to guide your clinical sails. Addressing stereotypes head-on, learning on the spot, dealing with complex identities in your patients, and always practicing with compassion will lend itself to staying ahead and staying fulfilled.
Pretty heavy stuff right? But there’s something else that caught my attention in reflection on the TEDx talk… I’ve searched the TED library of videos, and while there are plenty of doctors, scientists, and pioneers in research discussing medical ideas, I haven’t seen any medical laboratory scientists. If you find any, please correct me. But, as I understand it, it’s just me. And that’s something special.
There’s a culture shift in our profession, and a lot of us are talking about it. Pathology and laboratory medicine are stepping out from behind the healthcare curtain and asserting itself as a champion for patients, truth, and the importance of data-driven medicine. Not only do I talk to groups of folks every time I get a stage, but I use social media to reach clinicians and patients! Yes, I’m one of few medical students-turned-residency applicants who didn’t change their name to hide their online presence for the winter. But instead of a secret twitter hibernation, I’ve used social media as a tool to network, engage, and connect.
One of my favorite new projects is something I call #PathDoodles where I break down the aspects of pathology and some specialty topics for those outside of medicine (and sometimes just outside our profession). I’ve already covered things like “what is pathology?” and the importance of autopsies, the role of medical laboratory scientists, and I continue to add more regularly!

There’s a culture shift in our profession, and a lot of us are talking about it. Pathology and laboratory medicine are stepping out from behind the healthcare curtain and asserting itself as a champion for patients, truth, and the importance of data-driven medicine. Not only do I talk to groups of folks every time I get a stage, but I use social media to reach clinicians and patients! Yes, I’m one of few medical students-turned-residency applicants who didn’t change their name to hide their online presence for the winter. But instead of a secret twitter hibernation, I’ve used social media as a tool to network, engage, and connect.
One of my favorite new projects is something I call #PathDoodles where I break down the aspects of pathology and some specialty topics for those outside of medicine (and sometimes just outside our profession). I’ve already covered things like “what is pathology?” and the importance of autopsies, the role of medical laboratory scientists, and I continue to add more regularly!
Follow me on Twitter (@CEKanakisMD) and check out my TEDx talk:

–Constantine E. Kanakis MD, MSc, MLS (ASCP)CM completed his BS at Loyola University Chicago and his MS at Rush University. He writes about experiences through medical school through the lens of a medical lab scientist with interests in hematopathology, molecular, bioethics, transfusion medicine, and graphic medicine. He is currently a 2020 AP/CP Residency Applicant and actively involved in public health and education, advocating for visibility and advancement of pathology and lab medicine. Follow him on Twitter @CEKanakisMD