Case History
A 5 month old girl was brought to her pediatrician by her mother for redness of the medial canthus of her left eye. There was some associated thick discharge as well. The mother mentioned that her daughter had excessive tearing from the left eye since birth. The pediatrician diagnosed dacryocystitis, and prescribed a course of oral cefdinir. However, the swelling and redness continued to worsen, and the infant began to have low-grade fevers. She was sent to pediatric ophthalmology for a consult, and admitted to the hospital on IV clindamycin. The following day, she was taken to the operating room, where they opened the obstructed nasolacrimal duct and sent the contents for culture.
Laboratory Work-Up
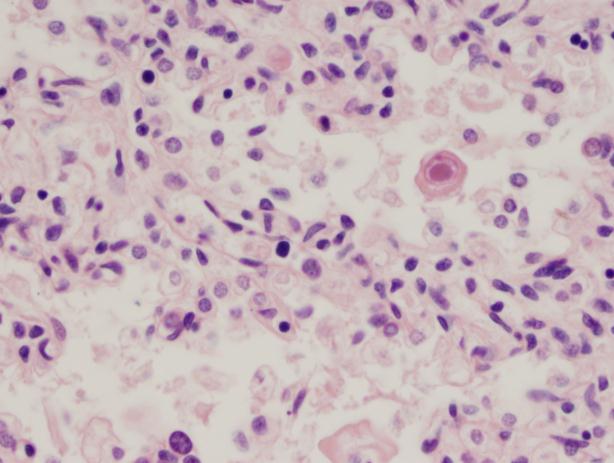
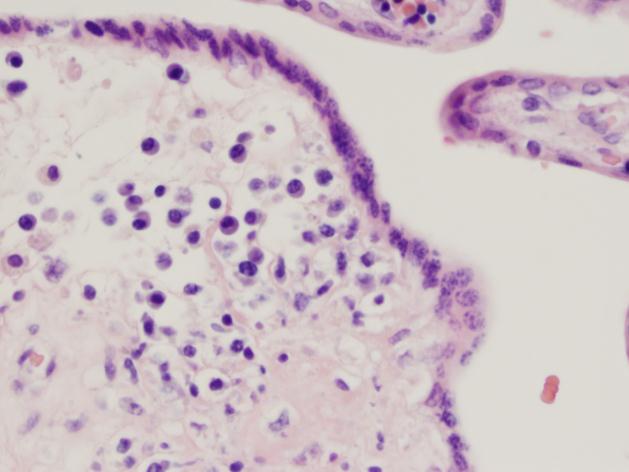
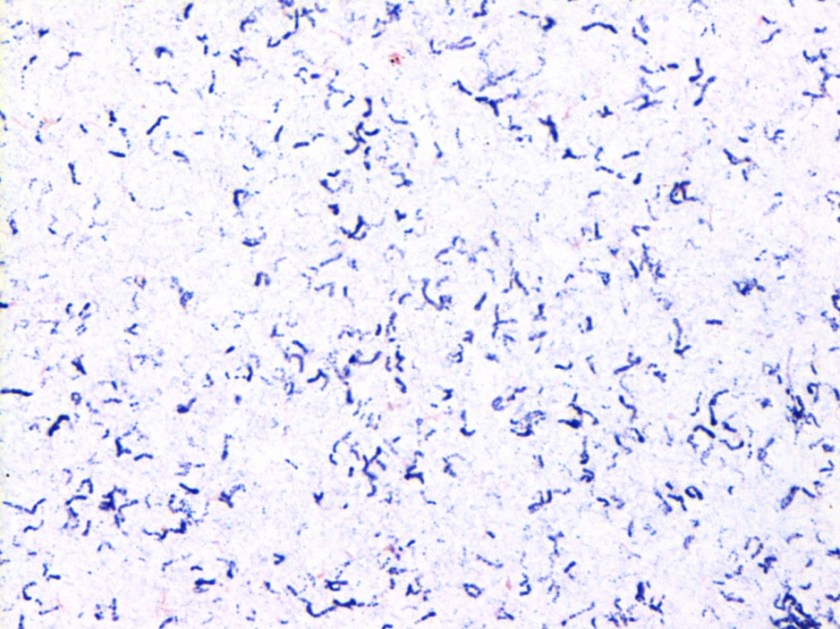
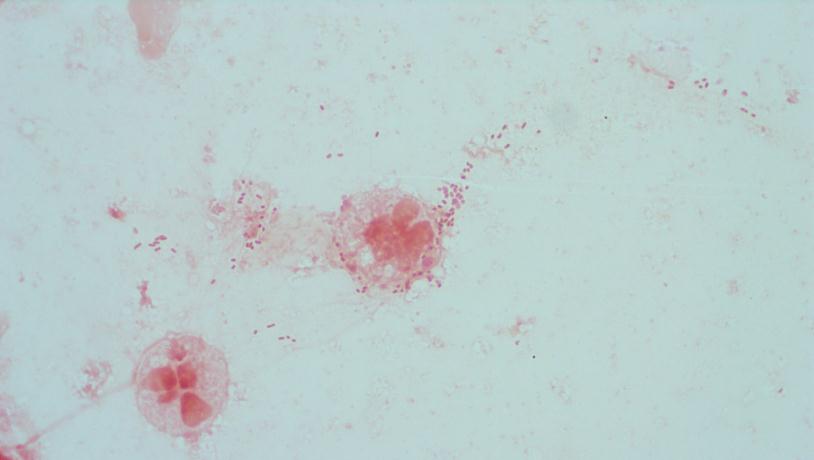
Gram stain and smear: No neutrophils, no bacteria seen.
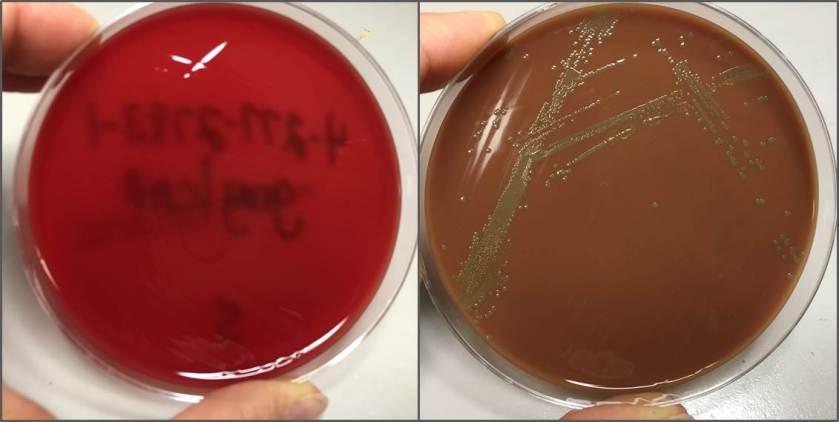
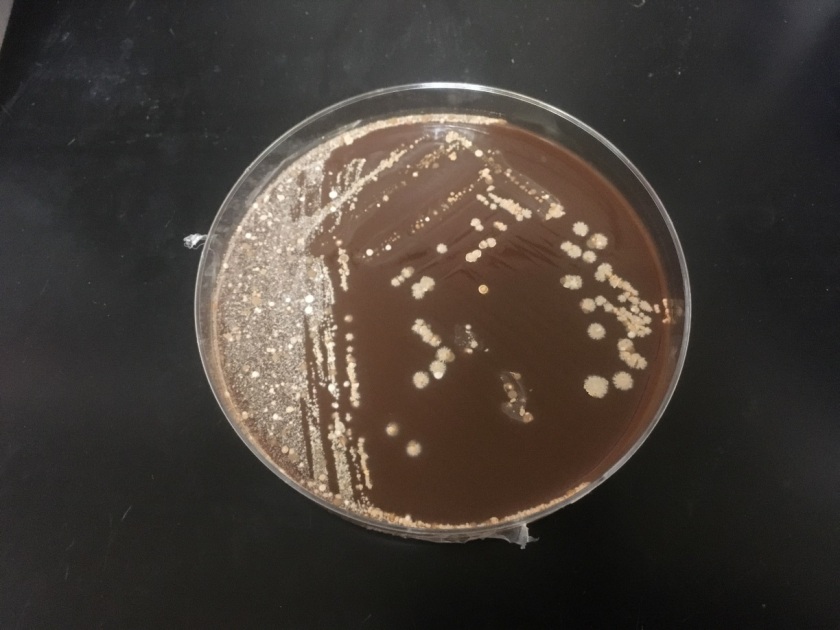
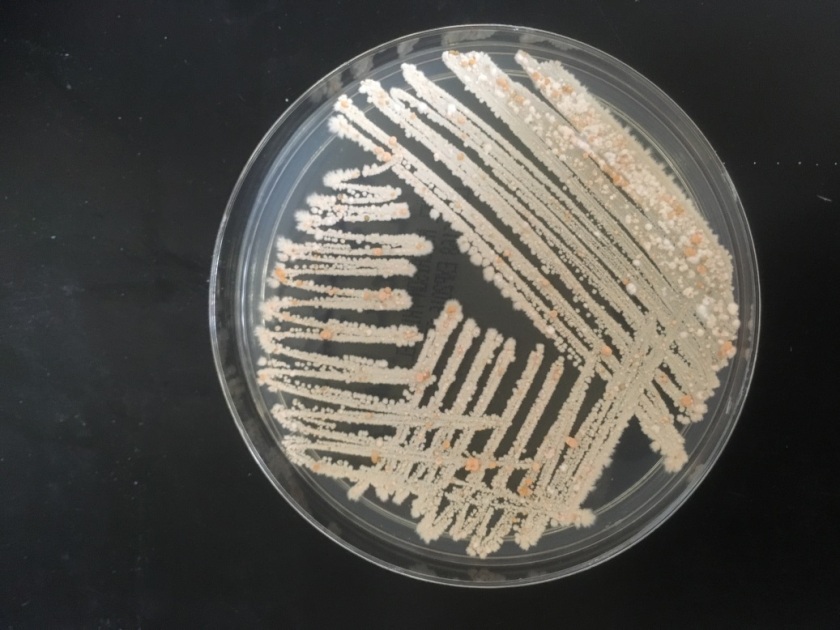
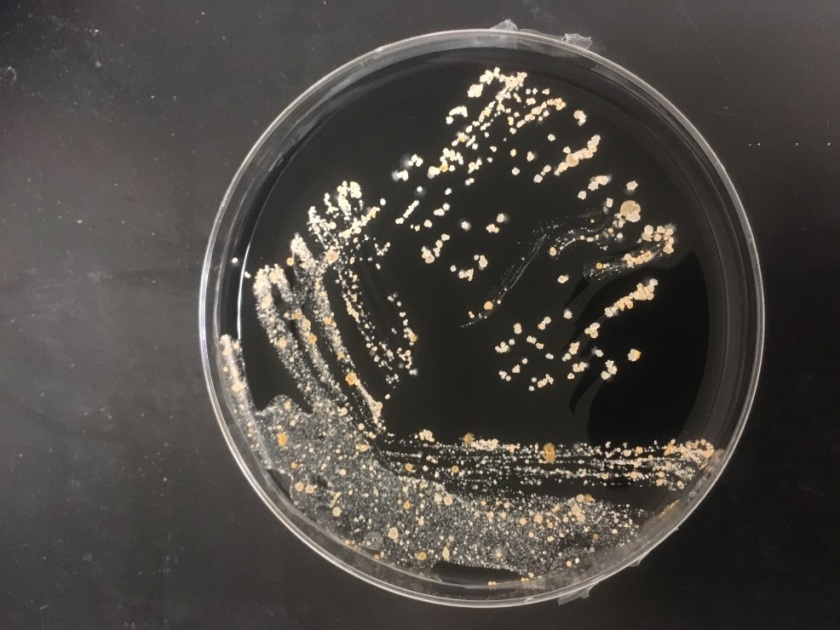
Routine bacterial culture showed growth of small, smooth and translucent colonies on the chocolate agar only. The colonies had a distinct wet-mouse odor.
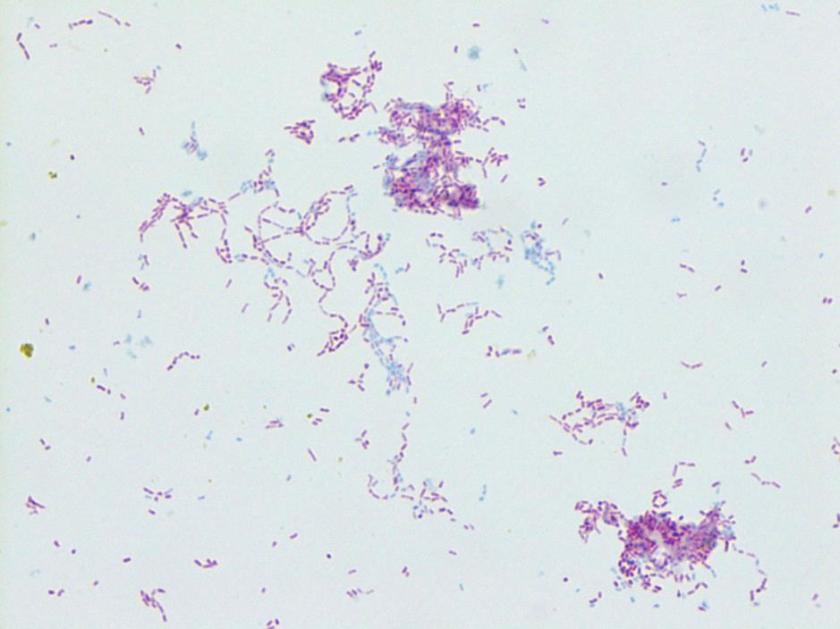
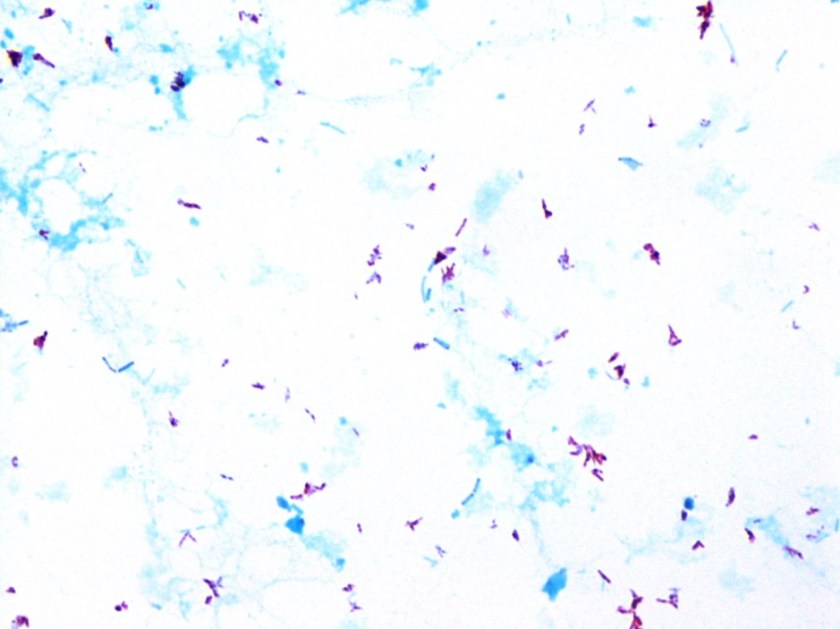
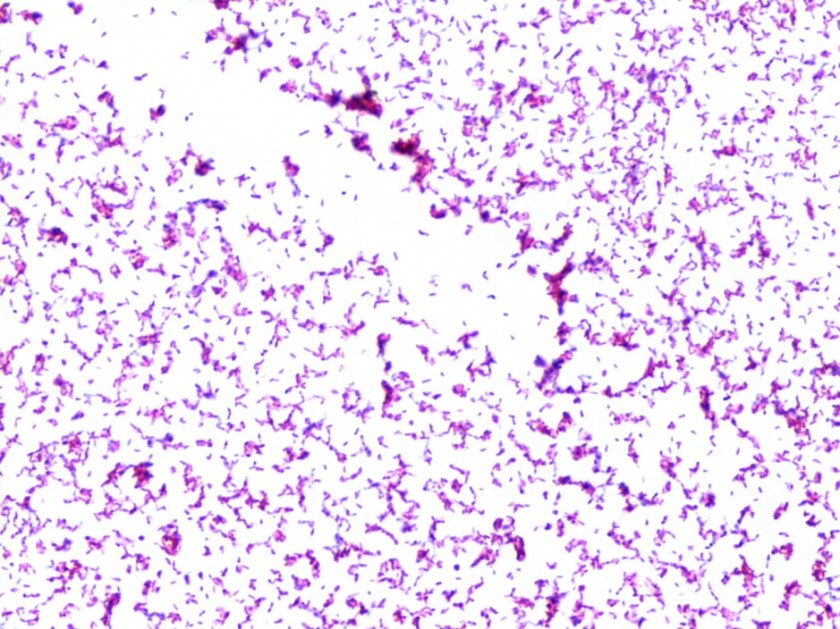
Repeat review of the gram stain showed moderate neutrophils, with gram negative coccibacilli both extracellular and intracellular.


Discussion
The gram stain and colony morphology described above are consistent with Haemophilus influenzae. Identification of the organism was confirmed by MALDI-TOF.
Congenital nasolacrimal duct obstruction is a common problem, affecting up to 6% of newborns. The vast majority of cases will resolve without treatment by the time the child is 6 months old. However, complications can arise, include acute or chronic dacryocystitis. Acute dacryocystitis causes swelling, warmth, and erythema with or without purulent discharge. The organisms most commonly implicated are alpha-hemolytic streptococci, Staphylococcus epidermidis, and Staphylococcus aureus. In chronic dacryocystitis, there is purulent drainage from the eye but no other signs or symptoms. The most common organisms isolated in these cases are Streptococcus pneumonia, Haemophilus influenzae, Pseudomonas aeruginosa, and viridans group Streptococci. Acute dacryocystitis usually requires systemic antibiotics, while chronic can be treated with topical antibiotics. In this case, the infant had clinical features of acute dacryocystitis, but infection with an organism more typically associated with chronic dacryocystitis.
H. influenzae is a gram negative coccobacillus, which grows only on chocolate agar due to its requirement for factors V and X. However, H. influenzae can grow on blood agar if it is growing around an organism that hemolyzes the red blood cells in the media, releasing factor V (i.e. Staphylococcus aureus), a phenomenon known as satelliting.
As illustrated by this case, reviewing gram smears inconsistent with the final culture is an important aspect of quality assurance in the microbiology laboratory. This allows the opportunity to provide continuous feedback to technologists on their technique, and lets us keep track of any trends or common mistakes that may be occurring. In this instance, on review of the gram smear it appears the original reader examined only the very thick portions of the smear, which were uninterpretable. However, by moving out to the edges, neutrophils and bacteria were clearly visible. Feedback was provided to the original reader. The provider was immediately called and notified that a corrected report would be issued; the patient was switched to oral Augmentin based on these results.
-Alison Krywanczyk, MD is a 3rd year anatomic and clinical pathology resident at the University of Vermont Medical Center.

-Christi Wojewoda, MD, is the Director of Clinical Microbiology at the University of Vermont Medical Center and an Assistant Professor at the University of Vermont.