Case History
A 24 year old African American male presents to the emergency department after he lost consciousness and fell at home. Currently, he complains of a significant headache, double vision and confusion. He also states he has a cough and shortness of breath with exertion. Over the past month, he has generally felt unwell and reports recurrent subjective fevers, night sweats, a 20 pound unintentional weight loss and nausea with a loss of appetite. On physical exam he is found to be febrile (103.1°F), with coarse breath sounds over the right chest. His neurological exam is normal. Chest x-ray shows right lobe infiltrates and bilateral perihilar opacities. A lumbar puncture is performed and showed an elevated opening pressure (28 cm H2O) with an increased white cell count (58% neutrophils, 32% lymphocytes). Blood, sputum and CSF specimens are sent to the microbiology laboratory for Gram stain and culture.
Laboratory Identification
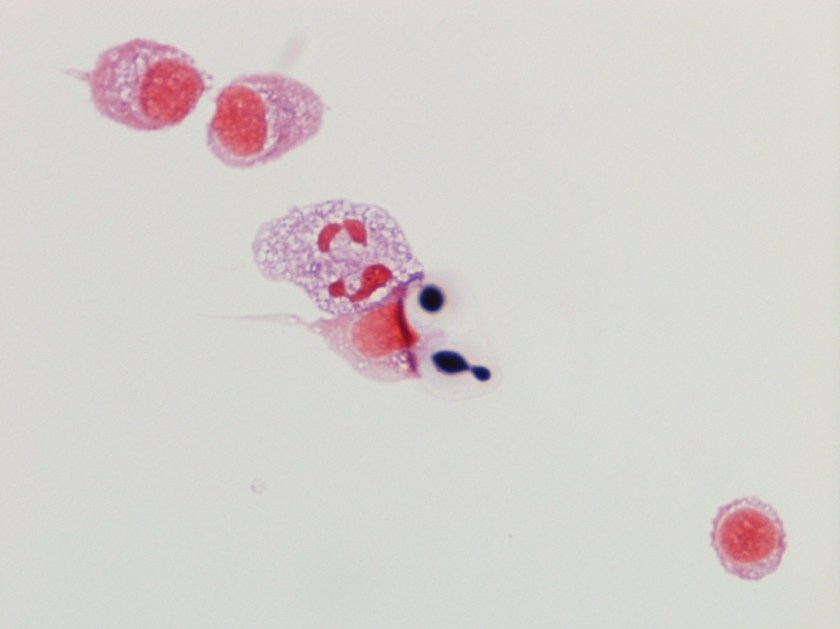
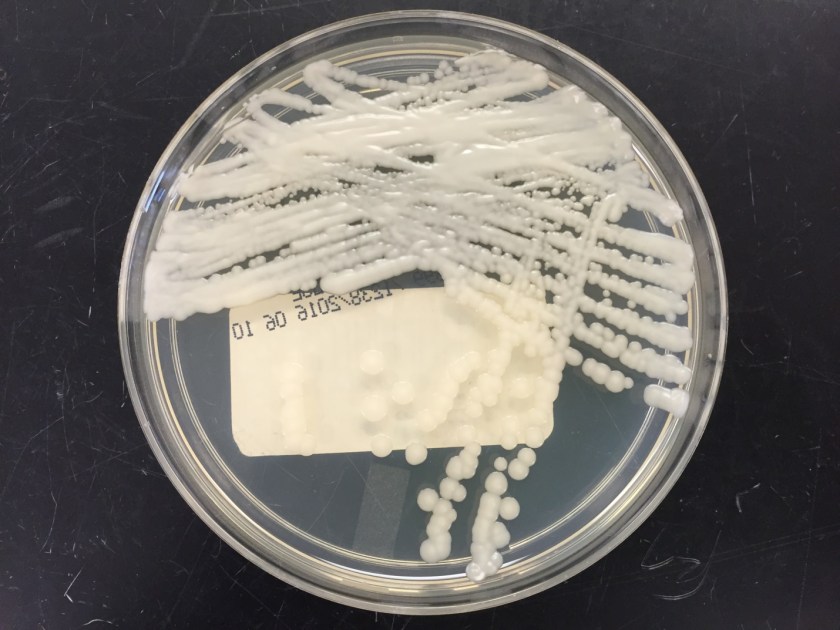
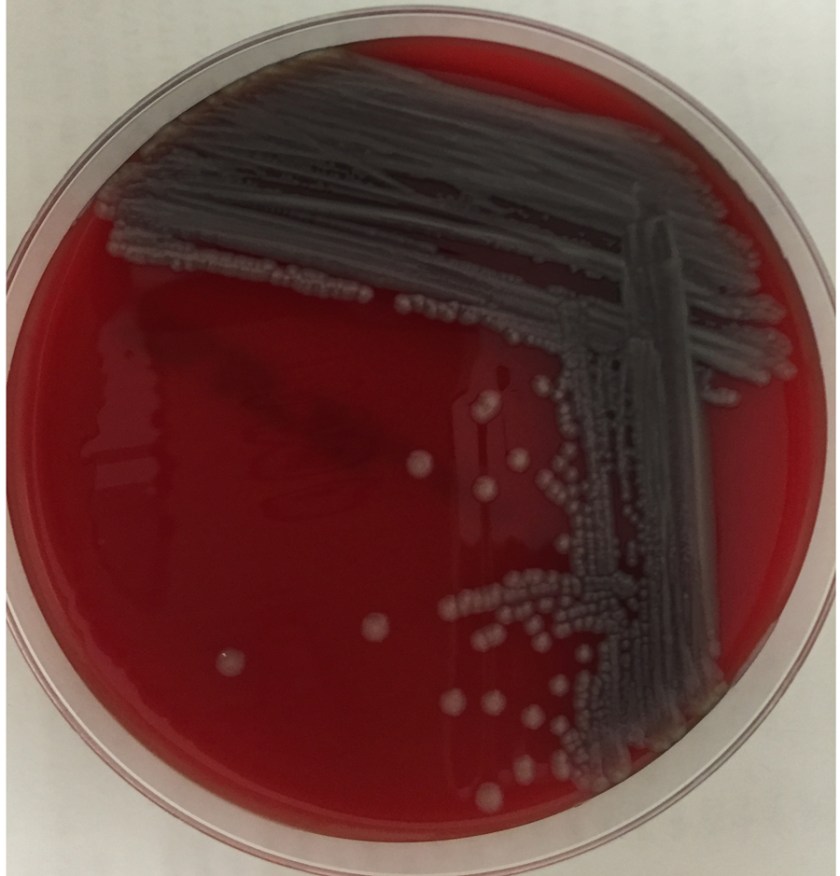
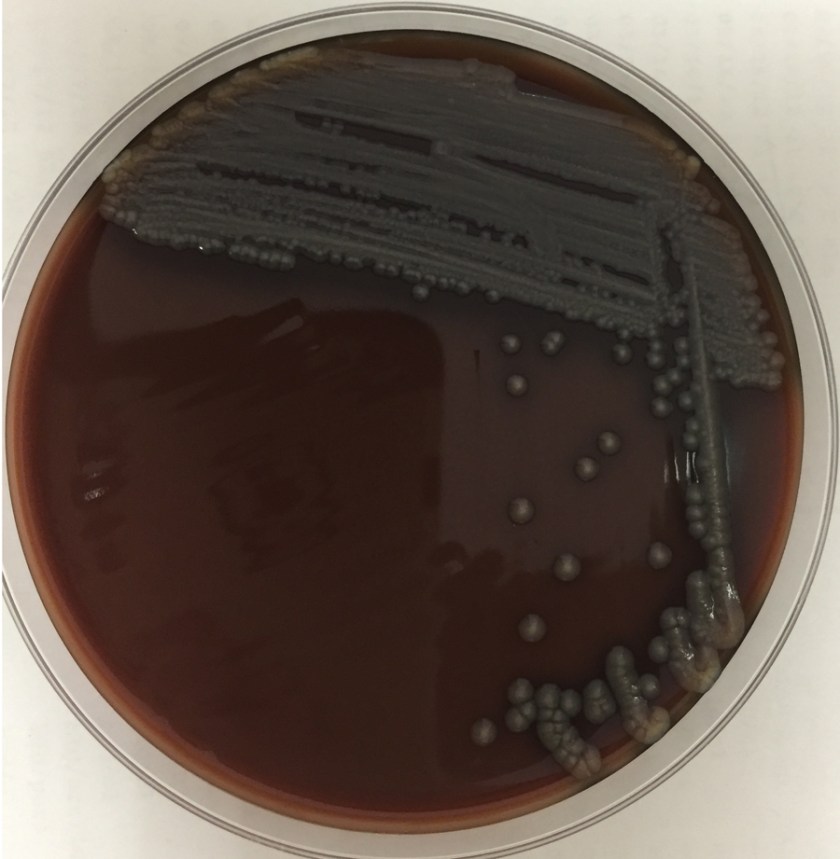
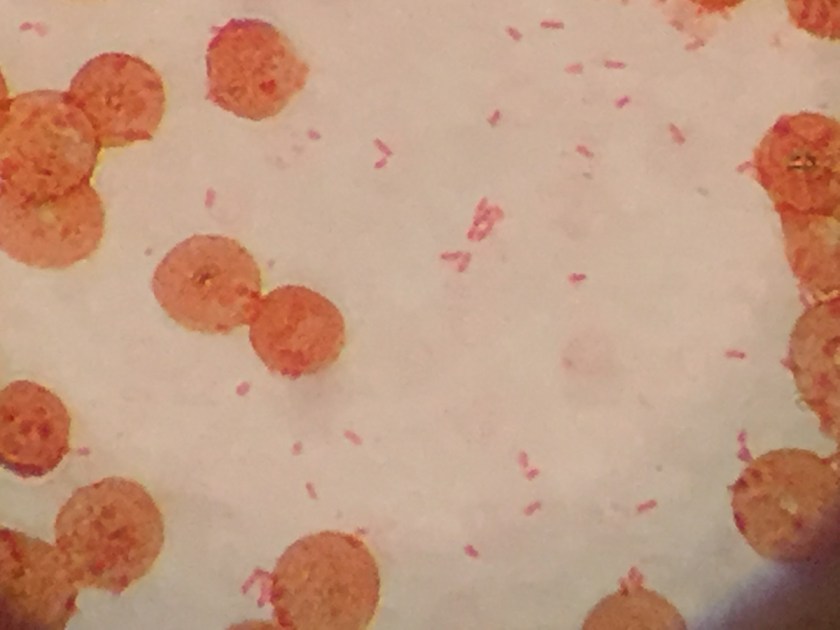
The centrifuged Gram stain of the CSF showed few yeast forms that exhibited narrow based buds and were surrounded by outline of a thick capsule (Figure 1). Bacterial and fungal culture of the CSF revealed a white, creamy yeast that grew after 2-3 days incubation (Figure 2). The organism was identified by MALDI-TOF as Cryptococcus neoformans. A cryptococcal antigen test was performed on the CSF and showed a titer of 1:1024. In addition, C. neoformans grew from 4/4 of the patient’s blood cultures. While waiting for the culture results, the patient was found to be HIV positive for a viral load of 975,882 vc/ml and an absolute CD4 count of 17 cells/cm2. No other significant pathogens grew from any of the other cultures obtained.
Discussion
Cryptococcus neoformans is an encapsulated yeast found widely in nature. It is typically found in soil that is contaminated with pigeon droppings or bat guano that has a high nitrogen content allowing the organisms to proliferate. C. neoformans is acquired via the inhalational route particularly when dust is generated. Individuals with a higher risk of infection include those who work in poultry farms, excavators and spelunkers. In addition, immunosuppressed people, especially with cellular immunodeficiencies such as HIV, those with hematopoietic malignancies and those taking immunosupressants have an increased risk of acquiring infection with C. neoformans. While the organism commonly causes pulmonary infections, fungemia and disseminated disease with cutaneous involvement have been reported. C. neoformans has a particular tropism for the central nervous system and often initially presents as meningoencephalitis without evidence of disease elsewhere.
Historically, the first step in diagnosis of suspected meningitis due to Cryptococcus in the HIV era was to perform an India ink preparation on a CSF specimen. This test served to identify the variably sized (2-20 µm), narrow based budding yeast forms due to the prominent capsule not allowing the ink to reach the cell wall of the organism and creating a halo like appearance around the yeast. While this test provided a rapid diagnosis and was inexpensive to perform, its lack of sensitivity has paved the way for detection of the cryptoccocal polysaccharide antigen via a latex agglutination method. This test can be performed on serum & CSF specimens and provides both diagnostic (qualitative) and prognostic (quantitative) information. By following titers by this method throughout the disease course and therapy, clinicians can monitor response to treatment (declining titers) and relapsed infections (increasing titers).
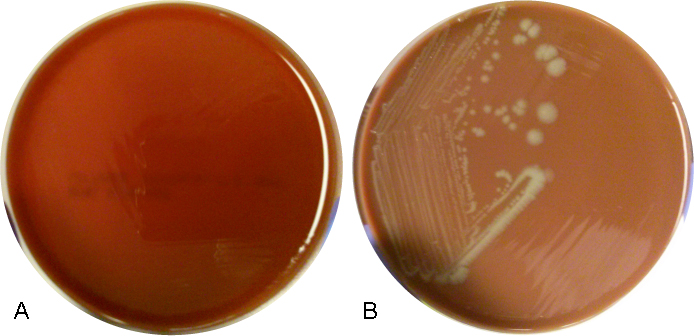
In culture, C. neoformans appears as white, creamy to mucoid colonies which grow well on Sabouraud dextrose agar within 3 days. It is positive for both urea and phenoloxidase, which is exemplified by the colony’s reddish-brown pigmentation on bird seed agar. The presence of pigment on this agar helps to differentiate C. neoformans and C. gatti from other cryptococcal species, a distinction that may be important therapeutically as the latter are often more resistant to standard treatments. Microscopically on cornmeal agar, C. neoformans shows variability in size and is uniformly spaced among each yeast form due to the presence of the thick capsule. This characteristic is described as resembling glass beads. No pseudohyphae are present. Other commonly employed laboratory methods currently used to identify C. neoformans include automated instruments, such as the Vitek, and MALDI-TOF mass spectrometry.
In the case of our patient, he received two weeks of induction with amphotericin B and flucytosine. He was discharged home on oral fluconazole maintenance therapy as well as bactrim and azithromycin for prophylaxis from other infectious organisms. It is important to note that C. neoformans is resistant to echinocandins and this group of antifungals should not be used for treatment.

-Tudor Vladislav, MD, is a 2nd year Anatomic and Clinical Pathology resident at the University of Mississippi Medical Center.

-Lisa Stempak, MD, is an Assistant Professor of Pathology at the University of Mississippi Medical Center in Jackson, MS. She is certified by the American Board of Pathology in Anatomic and Clinical Pathology as well as Medical Microbiology. Currently, she oversees testing performed in both the Chemistry and Microbiology Laboratories. Her interests include infectious disease histology, process and quality improvement and resident education.