Case History
A 2 week old African American female presented to the pediatric emergency department (ED) with erythema, swelling and copious mucopurulent discharge from the right eye. One week earlier, her Mom noted similar symptoms in the left eye which spontaneously resolved. Mom denied fever, irritability, lethargy, rash, and respiratory or urinary symptoms. The baby was born at term through a spontaneous vaginal delivery with no complications. Mom received regular prenatal care and all screening tests were negative. The baby received erythromycin eye ointment at birth prior to initial discharge. Complete blood count showed a slight leukocytosis (WBC 15.7 TH/cm2) and cerebral spinal fluid (CSF) values were unremarkable. A complete sepsis work up was performed with blood, CSF, eye swabs and urine sent for bacterial cultures. Given the high suspicion for a sexually transmitted infection, an eye swab was also collected for Neisseria gonorrhoeae and Chlamydia trachomatis polymerase chain reaction (PCR). Herpes simplex virus PCR from the CSF was also performed. The patient was started on IV ampicillin, cefotaxime and oral erythromycin in the ED.
Lab results
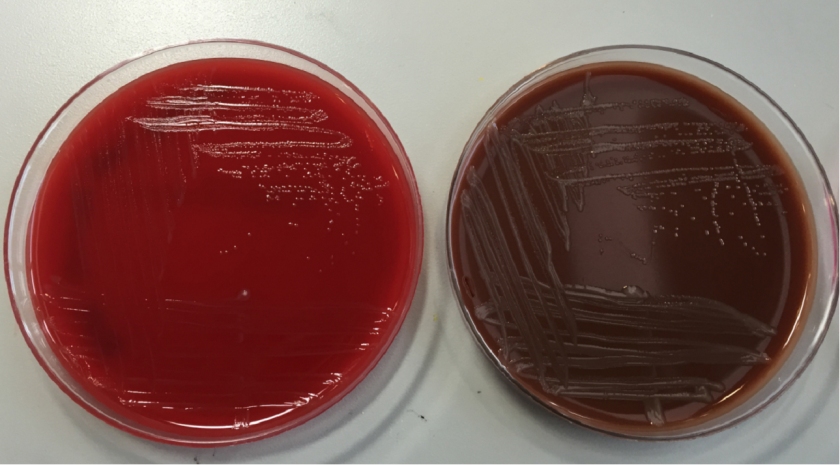
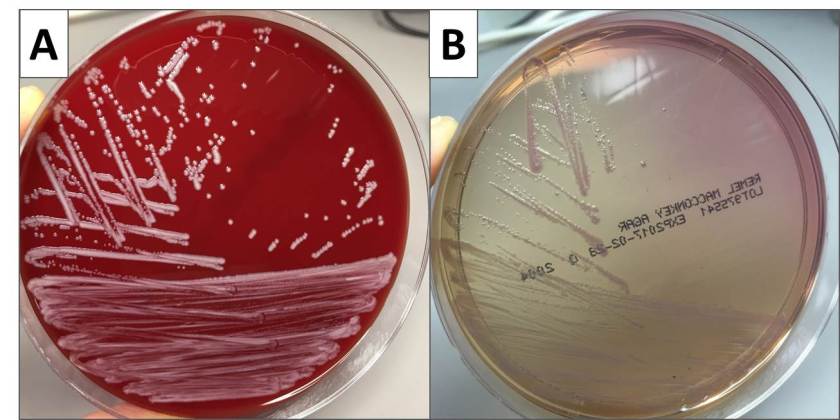
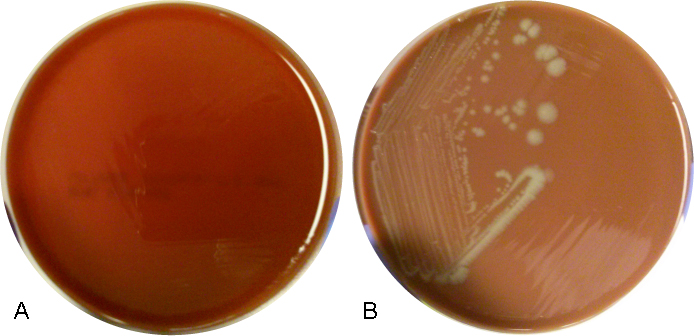
The organism was positive for both catalase and oxidase and identified by matrix-assisted light desorption ionization- time of flight (MALDI-TOF) as Neisseria meningitidis. The health department also confirmed the identification. PCR of the eye swab was negative for Neisseria gonorrhoeae and Chlamydia trachomatis. Bacterial cultures from the blood, CSF and urine were all negative.
Discussion
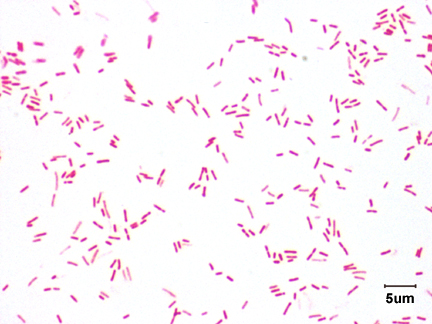
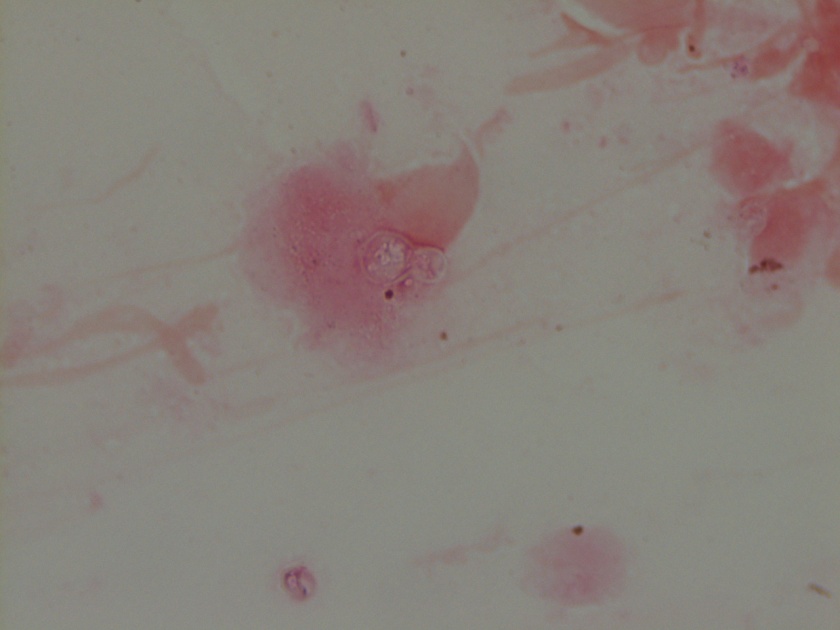
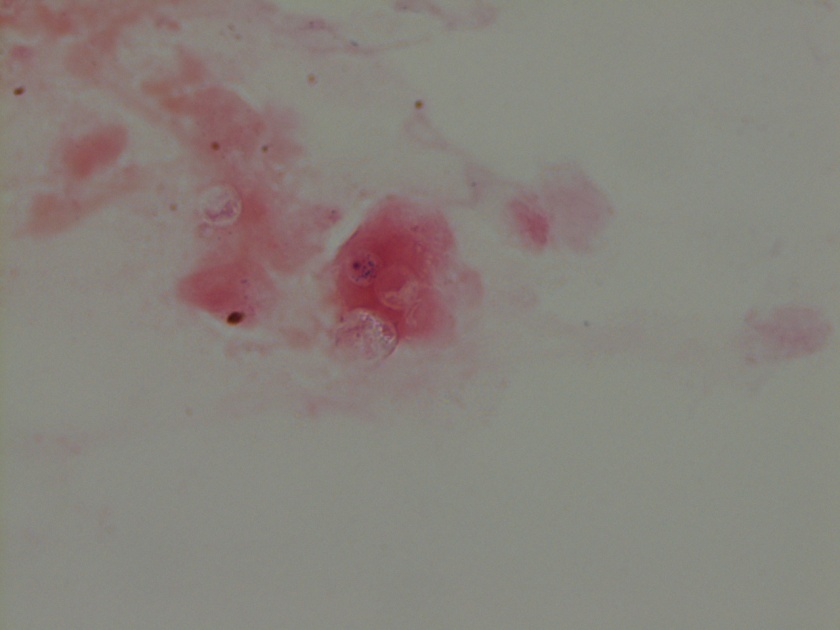
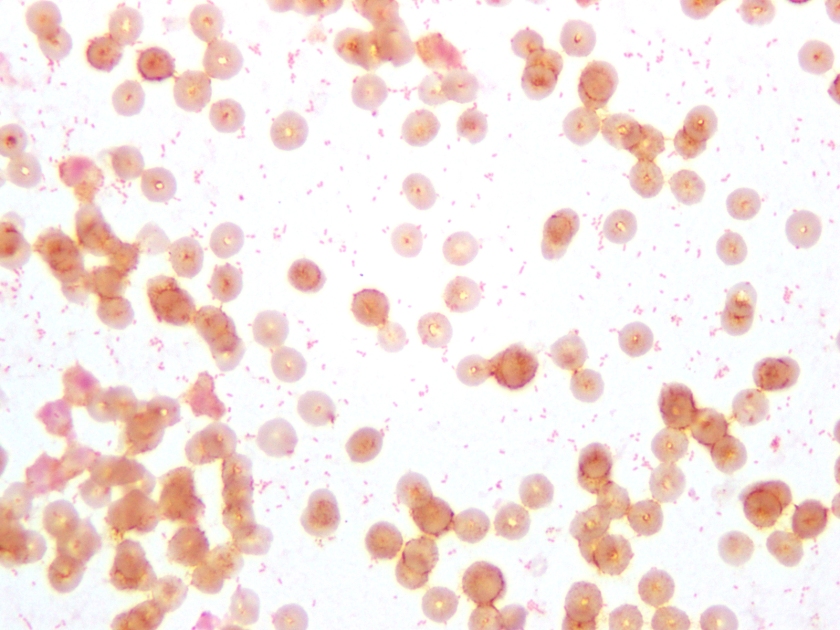
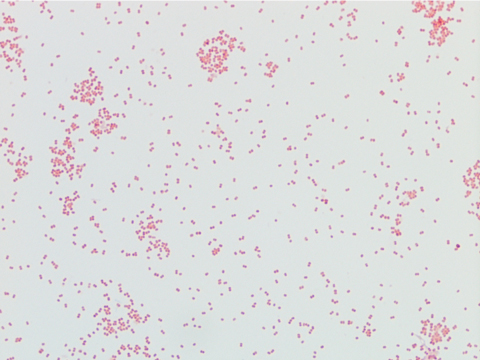
Neisseria meningitidis is an encapsulated Gram negative diplococcus (Image 2) that is usually transmitted through large droplet secretions from the oropharynx from colonized individuals. It can cause invasive meningococcal disease, which can present as meningitis (high fever, stiff neck, and headache), acute sepsis or a combination of both. Waterhouse Friderichsen-syndrome can result in severe dissemination forms of the disease and is characterized by petechial hemorrhages, involvement of the adrenal glands, and disseminated intravascular coagulopathy (DIC). Rarely, N. meningitidis can cause acute bacterial conjunctivitis (1.5 % – 2.5% of cases). Local complications, including corneal ulcers or a more systemic disease, may occur as well.
N. meningitidis produces multiple virulence factors that help cause disease and evade human immune defense mechanisms. The polysaccharide capsule represents the major virulence factor and is also the basis of meningococcal serotyping. Twelve different capsular serotypes can be distinguished, with serotypes A, B, C, W, X, and Y accounting for most invasive disease worldwide. Other virulence factors include pili, which helps the bacteria attach to host surfaces, and IgA protease, an enzyme that cleaves IgA and allows the bacteria to escape the humoral portion of the immune system.
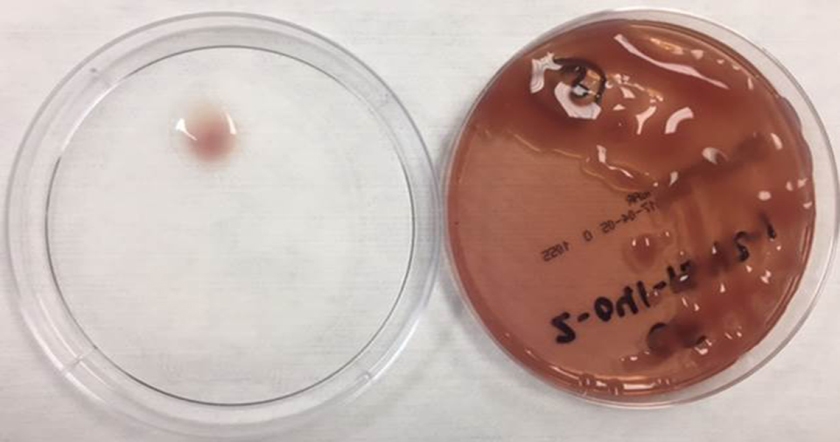
In the laboratory, N. meningitidis grows well on both blood and chocolate agars after 24 hours of incubation (Image 1) and it is positive for both catalase and oxidase. Traditionally, sugar fermentation was used to differentiate Neisseria species from one another. N. meningitidis ferments both glucose and maltose whereas N. gonorrhoeae is only capable of fermenting glucose. Currently, more rapid identification methods (MALDI-TOF, PCR and sequencing) are being increasingly used in most laboratories for a faster and more accurate identification of Neisseria species. The work up of suspected N. meningitidis isolates must be performed using BSL 2 standards, as aerosols created during mobilization from culture plates or performance of biochemical testing has been known to cause invasive disease in laboratory workers.
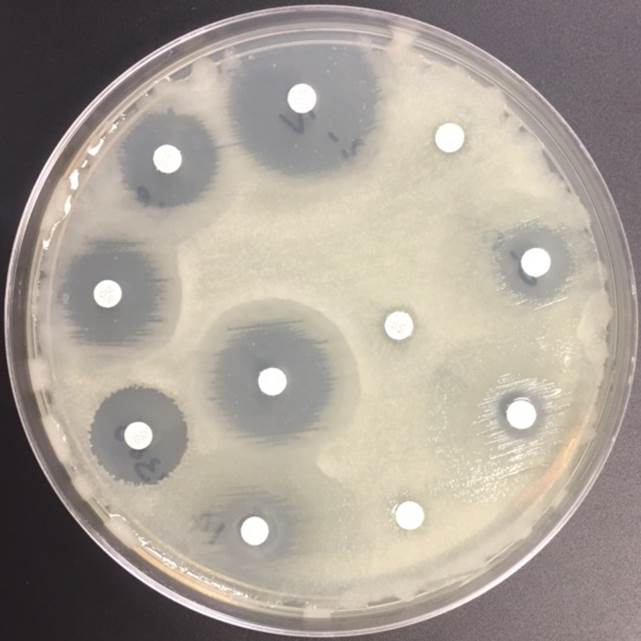
In general, N. meningitidis is susceptible to penicillin and cefotaxime, but susceptibility testing by disk or gradient diffusion is recommended. Both rifampin and ciprofloxacin can be used for chemoprophylaxis in close contacts of the patient and healthcare & laboratory workers. In addition, a number of meningococcal vaccines are available in the United States (US) and the Centers for Disease Control & Prevention (CDC) recommends vaccinating all adolescents and people at high risk for infection (college students, military recruits, those who had a splenectomy and patients with complement deficiencies). The most common vaccine is a quadrivalent polysaccharide-protein conjugate vaccine which covers serotypes A, C, W and Y. Recently in 2014, the Food and Drug Administration (FDA) approved Trumenba, a vaccine effective against serotype B, which a common serotype causing invasive disease in the US.

-Akram Shalaby, MD, is a first year anatomical and clinical pathology resident at the University of Mississippi Medical Center.

-Lisa Stempak, MD, is an Assistant Professor of Pathology at the University of Mississippi Medical Center in Jackson, MS. She is certified by the American Board of Pathology in Anatomic and Clinical Pathology as well as Medical Microbiology. She is the director of the Microbiology and Serology Laboratories. Her interests include infectious disease histology, process and quality improvement and resident education.