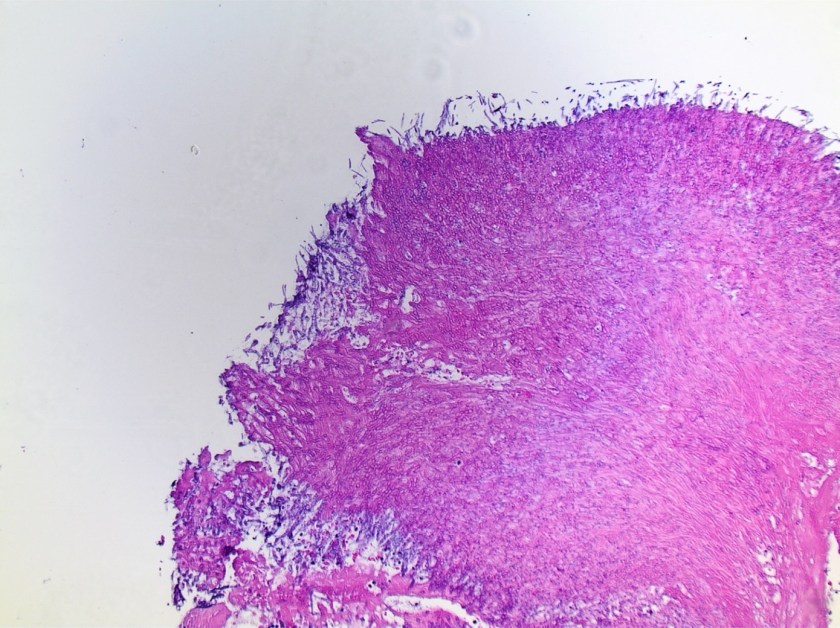
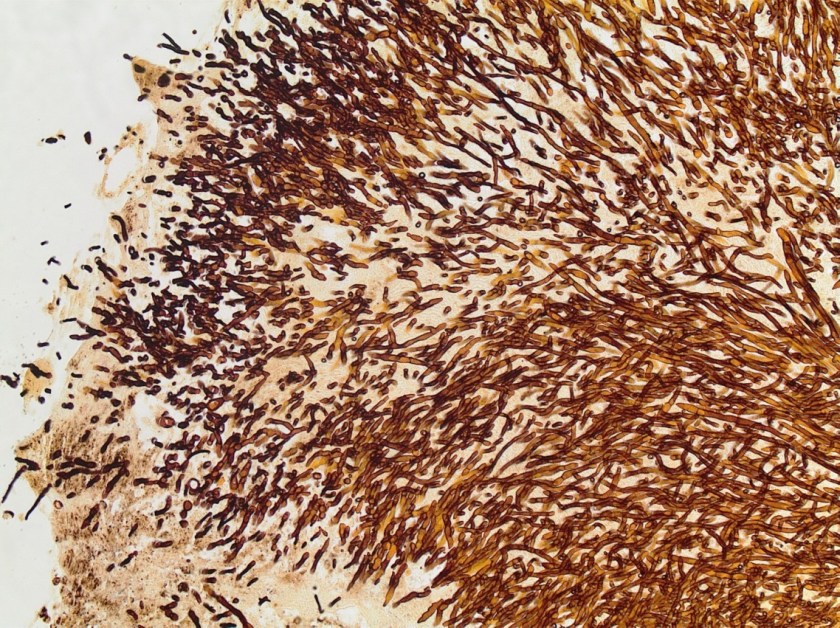
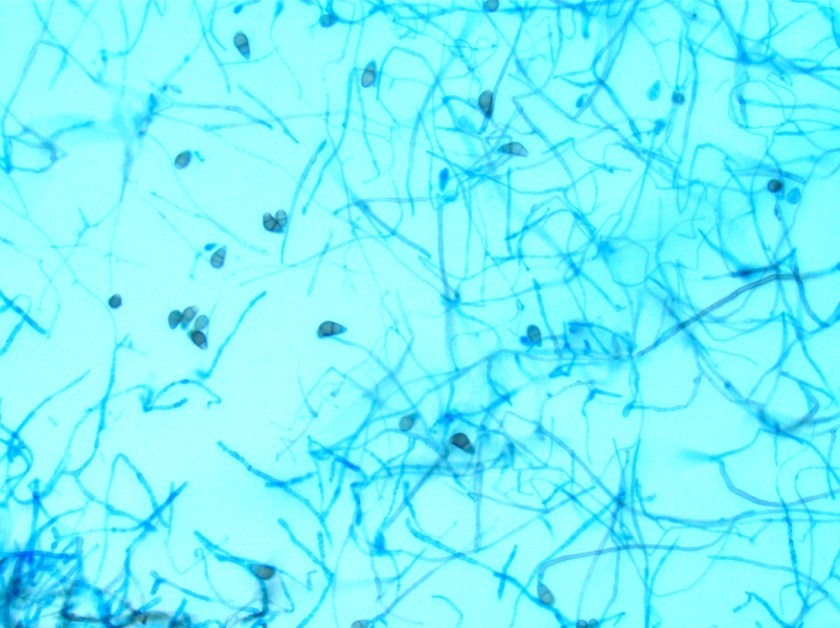
Our patient is a 47-year-old female with a history of type II diabetes mellitus, hypertension, pancreatic insufficiency, systemic sarcoidosis with lung and liver involvement. She was admitted into the ED for severe hypercalcemia, hypokalemia and hypomagnesemia. Her total calcium concentration was at 15.1 mg/dL (ref range: 8.3-10.2 mg/dL, critical: >13.0) at admission and albumin was low. Further testing revealed 25-hydroxy vitamin D (25(OH)D) of 23.2 ng/mL, which is considered insufficient, and decreased PTH of < 15 pg/mL (ref range: 15 – 65). From these results, primary hyperparathyroidism was ruled out. PTH related peptide (PTHrp) was tested given her sarcoidosis history.
PTHrp is produced by some cancers, especially kidney, breast and lung cancers, and as well as lymphoma and leukemia. It has the same N-terminal and binds to the same receptor as PTH, therefore sharing some functions of PTH. In patients with hypercalcemia associated with malignancy, PTHrP may be evaluated. There are also case reported sarcoidosis-related hypercalcemia due to production of PTHrp. In the case, PTHrp was normal at 0.4 pmol/L (ref range: < 2.0).
Further tests showed that 1, 25-dihydroxyvitamin D (DHVD) was elevated at a concentration of 93.3 pg/mL (ref: 18.0 – 78.0). In the presence of decreased 25(OH)D, this result suggested that the 1-alpha-hydroxylase could be the cause of hypercalcemia. DHVD is the active form of vitamin D. It promotes intestinal calcium absorption and, in concert with PTH, skeletal calcium deposition. 25(OH)D converts to DHVD via 1-alpha-hydroxylase, which is almost exclusively expressed in the kidney, but can also be found in some extrarenal tissues, including inflammatory cells of the monocyte/macrophage lineage commonly seen in sarcoidosis and other granulomatous diseases. DHVD produced in extrarenal tissues is PTH-independent, and moreover, elevated calcium induced by extrarenal DHVD can inhibit PTH production via calcium-sensing receptor (CaSR) on parathyroid cells.
Sarcoidosis is a multisystem inflammatory disease of unknown etiology manifests as granulomas found predominantly in the lungs and lymph nodes. Hypercalcemia is seen in about 10-13% of patients. Overproduction of 1-alpha-hydroxylase and production of PTHrp can both contribute to the hypercalcemia in some patients with sarcoidosis. In this case, PTHrp was normal and elevated 1-alpha-hydroxylase was found to be the cause of hypercalcemia.
In addition to treatment of the underlying disorder, treatment of hypercalcemia in sarcoidosis is aimed at reducing intestinal calcium absorption and DHVD synthesis. Besides dietary interventions, glucocorticoids and bisphosphonates have also been used successfully to treat hypercalcemia in sarcoidosis:
- Glucocorticoids: inhibit DHVD synthesis by the activated mononuclear cells (major contribution), inhibit intestinal calcium absorption and osteoclast activity
- Bisphosphonates: inhibit the resorption of bone by osteoclasts
References
- https://emedicine.medscape.com/article/301914-overview
- https://www.uptodate.com/contents/hypercalcemia-in-granulomatous-diseases
- https://www.uptodate.com/contents/diagnostic-approach-to-hypercalcemia
- https://www.ncbi.nlm.nih.gov/books/NBK109831/

-Rongrong Huang, PhD is a first year clinical chemistry fellow at Houston Methodist Hospital. Her interests include general clinical chemistry, genetic biochemistry and applications of mass spectrometry in clinical laboratories.

-Xin Yi, PhD, DABCC, FACB, is a board-certified clinical chemist, currently serving as the Co-director of Clinical Chemistry at Houston Methodist Hospital in Houston, TX and an Assistant Professor of Clinical Pathology and Laboratory Medicine at Weill Cornell Medical College.