Case History
A 65 year old male with a history of systolic heart failure secondary to non-ischemic (alcohol-induced) dilated cardiomyopathy underwent cardiac transplantation on 10/11/2016. He was hospitalized between 3/1/17 and 4/15/17 for neutropenia and CMV viremia. Two days after discharge, he presented to the hospital with a gradual-onset of left-sided sharp chest pain described as “soreness” over his rib cage and exacerbated by breathing. Associated symptoms included fever, malaise, and fatigue. In the emergency department, vital signs included: BP 144/75 mmHg, T 40.2°C, RR 24/min, HR 101 bpm, SpO2 97% on room air. A CBC revealed a normal white blood cell count and a chest X-ray demonstrated a lingular opacity. The immunocompromised patient was admitted for sepsis secondary to presumed pneumonia following recent hospitalization. He was treated empirically for hospital-acquired pneumonia with vancomycin and piperacillin-tazobactam. After a urine antigen test detected the presence of Legionella pneumophila serogroup 1, antibiotic treatment was changed to levofloxacin and an induced sputum culture was obtained for Legionella surveillance.

Discussion
Legionnaires’ disease, caused by Legionella bacteria, is a cause of 1-9% of both community-acquired and hospital-acquired pneumonias. Symptoms of fever, chills, cough, and chest pain are similar to other causes of pneumonia; however multiple organ systems may be involved, producing additional symptoms including gastrointestinal (diarrhea, nausea, and vomiting) and central nervous system (headache and confusion) findings. Legionella was first discovered after a 1976 outbreak of pneumonia among Pennsylvania State American Legion members who attended a convention at a Philadelphia hotel that had infected water in the air conditioning system; it is reported that 29 out of 182 infected people died. At present, the mortality rate of Legionnaires’ disease ranges from less than 10% in treated community-acquired cases to approximately 30% for hospital-acquired cases.
The genus Legionella contains greater than 60 species of which approximately 20 are human pathogens. Legionella pneumophila (consisting of serogroups 1-16) is the most common cause of Legionnaires’ disease and, in particular, L. pneumophila serogroup 1 causes 70-90% of cases. The organisms are ubiquitous in nature, particularly in warm freshwater environments including lakes and streams, where they infect and multiply within single-celled host organisms. Of pathogenic concern, they can be present in high numbers in human-made complex water systems (such as cooling towers, whirlpool spas, humidifiers, and decorative fountains). After environmental aerosols are inhaled or contaminated water is aspirated into the lungs, alveolar macrophages are infected by the obligate intracellular bacteria. Host risk factors for developing Legionnaires’ disease include organ transplantation, immunocompromised state, immunosuppresion, age greater than 60 years, chronic lung disease, and smoking.
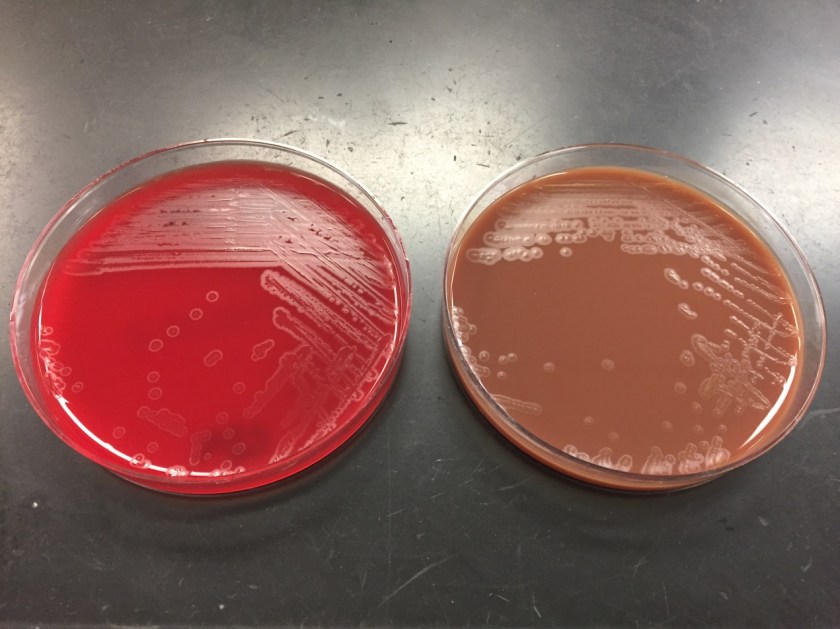
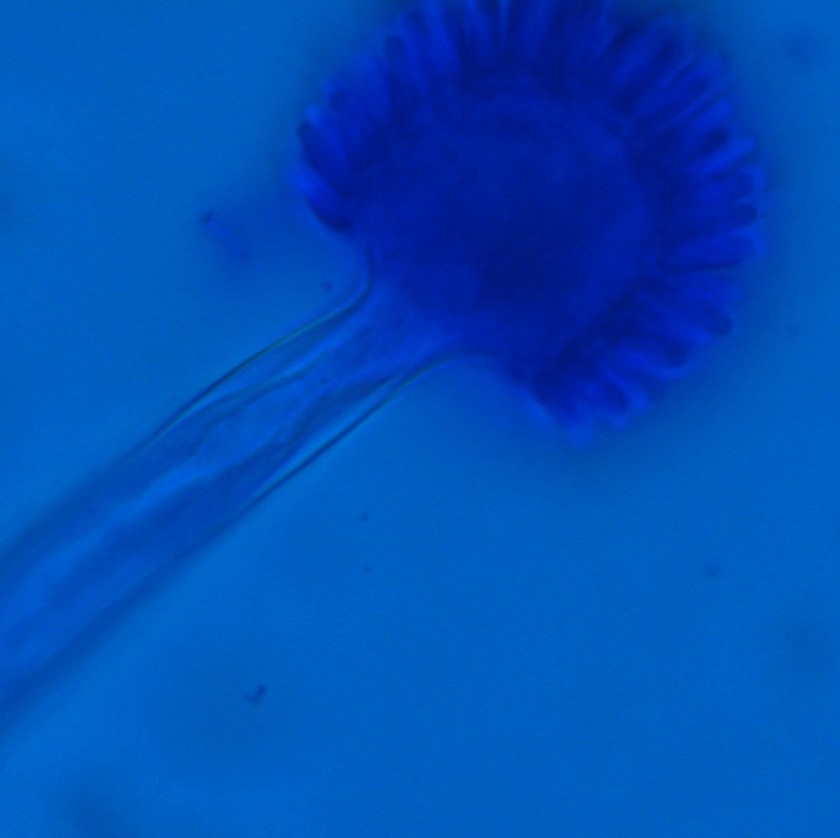
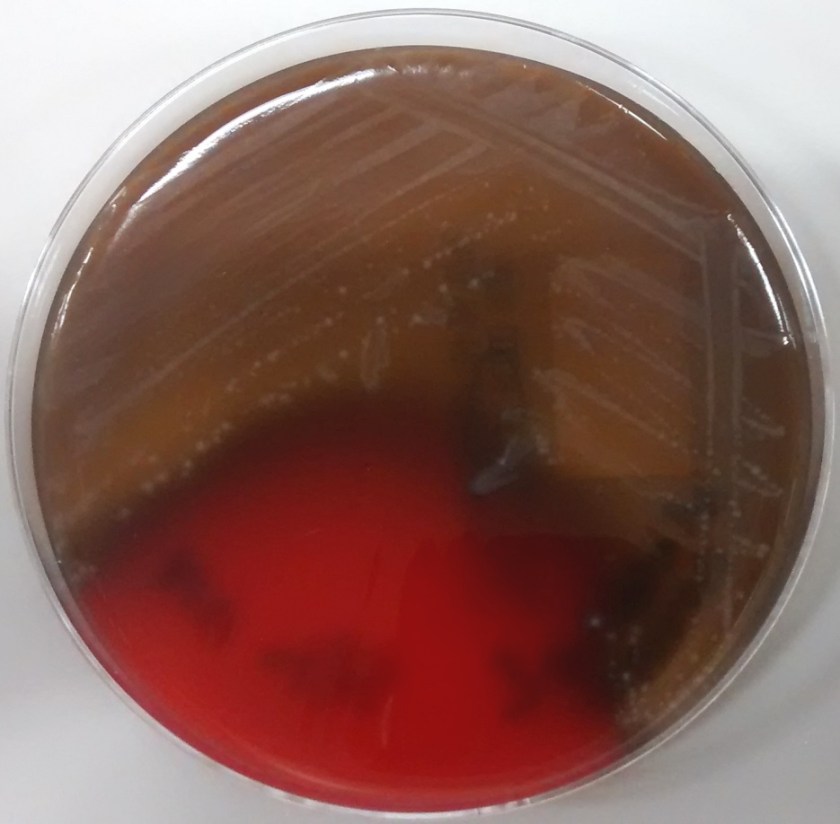
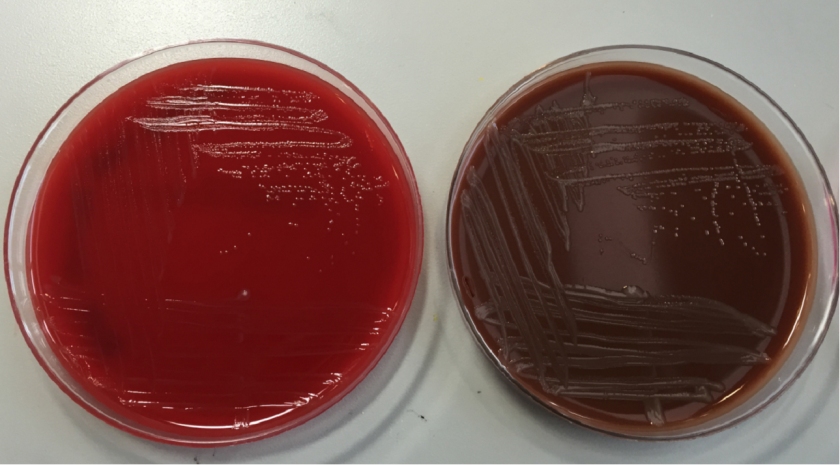
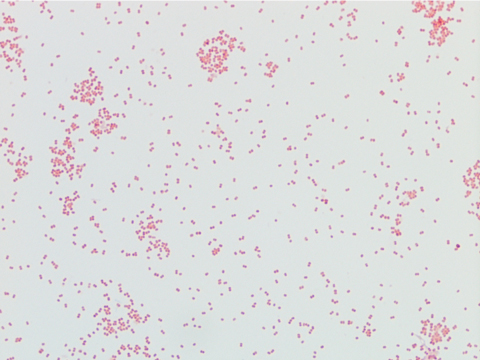
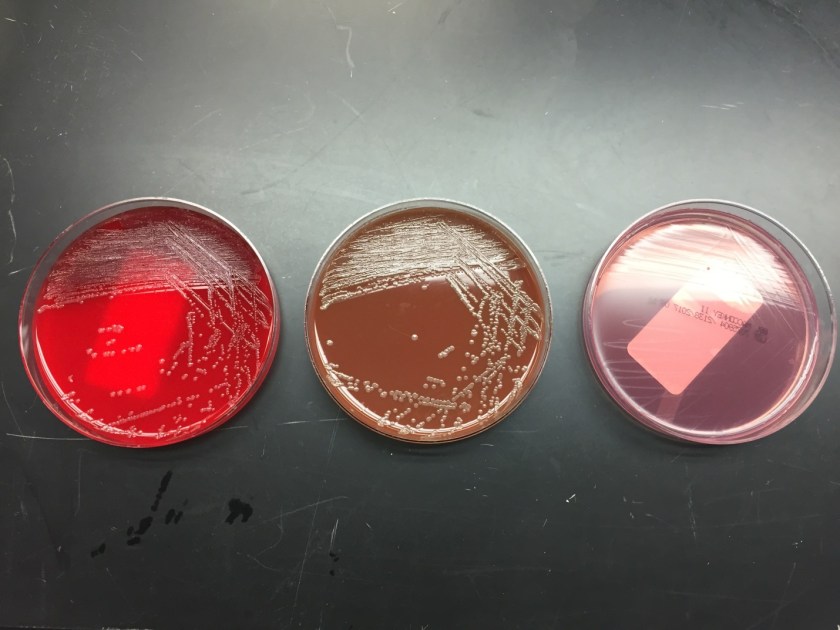
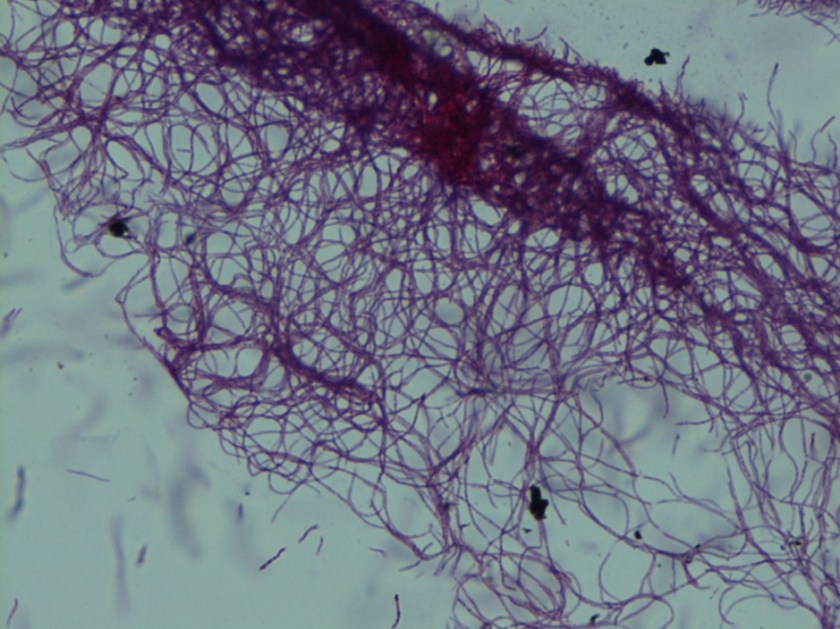
In the microbiology laboratory, Legionella are mesophilic (20-45 °C) obligate aerobes. The small, thin gram negative rods react poorly with Gram stains and are not usually stained in direct clinical samples. The patient’s Gram smear revealed moderate neutrophils, few squamous epithelial cells, and mixed gram positive and gram negative organisms present. Sensitivity for detecting the biochemically inert and fastidious bacteria is increased with culture on buffered charcoal yeast extract (BCYE) agar. For sputum samples that are likely contaminated with usual oropharyngeal flora, BCYE agar with polymyxin B, anisomycin, and vancomycin (PAV) media are used. After 3-5 days of incubation, Legionella colonies appear convex, circular, 3-4 mm in diameter, grey-white to blue-green, and glistening. This identification was confirmed by MALDI-TOF MS. Laboratory in vitro susceptibility studies are not recommended on individual isolates, as they do not correlate with clinical responses. Monotherapy with a fluoroquinolone (Levofloxacin) or macrolide (Azithromycin) is active against Legionella.
-Adina Bodolan, MD is a 1st year anatomic and clinical pathology resident at the University of Vermont Medical Center.

-Christi Wojewoda, MD, is the Director of Clinical Microbiology at the University of Vermont Medical Center and an Associate Professor at the University of Vermont.