The patient is a 67 year old male who first visited his dentist at the end of December complaining of pain in the jaw that he had been experiencing since early Dec. He had put off making an appointment because he didn’t want to have to go to the doctor with COVID precautions, but the pain was now radiating to his teeth, so he made a dentist appointment. The dentist found no evidence of abscess or other infection but ‘adjusted his bite’. The patient was advised to take over the counter NSAIDs as needed or pain but no prescriptions was needed. Three weeks later the patient visited an urgent care because he had no improvement of the jaw pain. At this time he relayed symptoms of cough, fever, chills, night sweats and chronic fatigue. Patient history included an active lifestyle with vigorous aerobic exercise several times a week, but the he stated that he had been feeling too fatigued to exercise for over a month. On exam the patient was found to be tachycardic with bilateral tonsillar lymphadenopathy and oropharyngeal exudate. The patient was tested for COVID, influenza and Group A Strep. The COVID-19 was negative, as was the influenza A and B, but the Group A Strep was positive. The patient was sent home with a prescription for antibiotics.
One week later, the patient called his PCP because he still had cough, fever and chills and now was experiencing shortness of breath. The office directed the patient to go to the ER but the patient was reluctant to go to the hospital and stated he would rather be seen at the office. On review of the patients chart, the PCP agreed to see him in the office because he had had a negative COVID test in the past week. Two days later the doctor examined the patient in his office and still suspected COVID-19. He ordered a PCR COVID-19 test along with CBC/differential and erythrocyte sedimentation rate (ESR). We received a routine CBC on the patient. Results are shown below.

The patient had no previous hematology or oncology history and no previous CBC received at our lab. The critical WBC was called to the physician. Based on the WBC and flags on the auto differential, a slide was made and sent to our CellaVision (CV). On opening the slide in CV, we immediately called our pathologist for a pathology review. A rare neutrophil was seen on the peripheral smear, with immature appearing monocytes, few lymphocytes and many blasts.
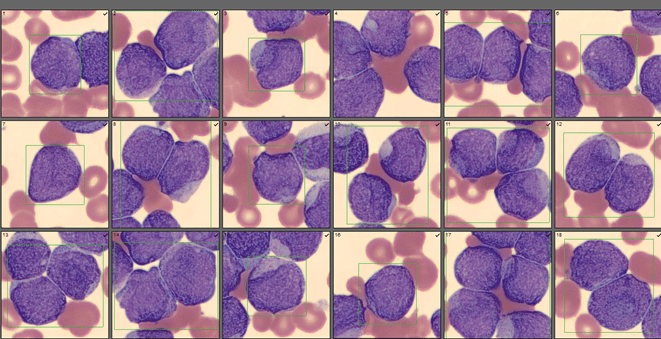

The pathologist reviewed the slide and the sample was sent for flow cytology studies and FISH. The pathologist’s comment ”Numerous blasts (>60%) consistent with Acute Myeloid Leukemia(AML). Specimen to be submitted for flow cytometry. Hematology consult recommended” was added to the report.


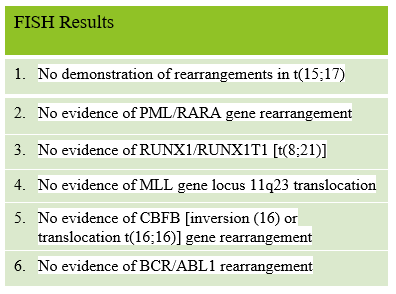
The myeloid/lymphoid disorders and acute leukemia analysis by flow cytometry reported myeloblasts positive for CD117,CD33, and CD13. Final interpretation was Acute Myeloid leukemia (non-M3 type).
AML is the most common form of leukemia found in adults. AML was traditionally classified into subtypes M0 through M7, based on the cell line and maturity of the cells. This was determined by how the cells looked under the microscope after a series of special staining techniques, but did not take into account prognosis. It is now known that the subtype of AML is important in helping to determine a patient’s prognosis. In 2016 World Health Organization (WHO) updated the classification system to better address prognostic factors. They divided AML into several broad groups, including AML with certain chromosomal translocations, AML related to previous cancer or cancer therapy, AML with involvement of more than one cell type, and other AML that don’t fall into the first three groups.2 Once a case has been placed in one of these broad groups, the AML can be further classified as poor risk, intermediate risk and better risk based on other test results. Better risk is associated with better response to treatments and longer survival.3 The European LeukemiaNET (ELN) first recommended integrating molecular and cytogenic data into classification to create such a risk classification system for AML in 2010 (ELN-2010). In 2017, this was again revised (ELN-2017) to further improve risk stratification. The ELN-2017 can be used to more accurately predict prognosis in newly diagnosed AML.1
What this means is that AML is now classified by abnormal cell type as well as by the cytogenetic, or chromosome, changes found in the leukemia cells. Certain chromosomal changes can be matched with the morphology of the abnormal cells. These chromosomal changes can help doctors determine the best treatment options for patients because these changes can predict how well treatment will work.
Examples of risk classification include the knowledge that some chromosome rearrangements actually offer a better prognosis. For example, a translocation between chromosomes 15 and 17 [t(15;17)] is associated with acute promyelocytic leukemia (APL or M3). APL is treated differently than other subtypes and has the best prognosis of all the AML subtypes. Other favorable chromosomal changes include [t(8;21)] and [inversion (16) or translocation t(16;16)]. Examples of intermediate risk prognosis are ones associated with normal chromosomes and [t(9;11)]. Poor prognosis is associated with findings such as deletions or extra copies of certain chromosomes or complex changes in many chromosomes.3
The patient was diagnosed with AML, non M3 type. AML prognosis is based on CBC results, markers on the leukemia cells (flow cytometry), chromosome (cytogenic) abnormalities found and gene mutations (molecular abnormalities). In this patient the FISH studies did not demonstrate any chromosome rearrangements, which alone would place him in an intermediate risk group. In addition, our patient was over age 60 and had a WBC over 100,000/mm3 which have both been linked to worse outcomes.
Here’s one more photo for your enjoyment! It’s not often that we see so many blasts in a patient with no previous history. As a side note, I was contemplating titling this blog “Fatigue and Shortness of Breath in the Time of COVID.” I can’t help but wonder if this patient would have been diagnosed 6-8 weeks earlier if this was another year and he had been seen when he first experienced symptoms. This year, emergency rooms and physicians have reported a decrease in numbers of patients being seen for chest pain, ketoacidosis, shortness of breath, strokes and other serious conditions. Many patients are reluctant or afraid of sitting in crowded waiting rooms, fearful they will catch COVID. And many doctors are only offering virtual visits or have reduced the number of patients being seen so it is harder to get appointments. This patient expressed his reluctance to seek medical help because of fears of COVID. He did not want to go out in public and waited almost a month for symptoms to go away on their own before first being seen. After going to the walk in center, he called his PCP a week later and was still averse to going to the ER as suggested by the doctor. Then he waited another 2 days for an office appointment. The doctor still suspected COVID, but fortunately for the patient, ordered a CBC. The flow cytometry and FISH studies were available the following day. The patient was referred for hematology consult but has not been seen again at our hospital.
References
- Boddu, P.C., Kadia, T.M., Garcia‐Manero, G., Cortes, J., Alfayez, M., Borthakur, G., Konopleva, M., Jabbour, E.J., Daver, N.G., DiNardo, C.D., Naqvi, K., Yilmaz, M., Short, N.J., Pierce, S., Kantarjian, H.M. and Ravandi, F. (2019), Validation of the 2017 European LeukemiaNet classification for acute myeloid leukemia with NPM1 and FLT3‐internal tandem duplication genotypes. Cancer, 125: 1091-1100. https://doi.org/10.1002/cncr.31885
- Mandel, Ananya. Acute Myeloid Leukemia Classification. Medical Life Sciences. https://www.news-medical.net/health/Acute-Myeloid-Leukemia-Classification.aspx
- Ari VanderWalde, MD, MPH, MA, FACP; Chief Editor: Karl S Roth, MD. Genetics of Acute Myeloid Leukemia. Medscape. Updated: Dec 17, 2018

-Becky Socha, MS, MLS(ASCP)CMBBCM graduated from Merrimack College in N. Andover, Massachusetts with a BS in Medical Technology and completed her MS in Clinical Laboratory Sciences at the University of Massachusetts, Lowell. She has worked as a Medical Technologist for over 40 years and has taught as an adjunct faculty member at Merrimack College, UMass Lowell and Stevenson University for over 20 years. She has worked in all areas of the clinical laboratory, but has a special interest in Hematology and Blood Banking. She currently works at Mercy Medical Center in Baltimore, Md. When she’s not busy being a mad scientist, she can be found outside riding her bicycle.

Very interesting case, mainly lhe clinical part which has an important massage and lesson to put good ddx and establish good investigation