ASCP’s 40 under Forty program recognizes forty pathologists, lab professionals, and residents under the age of 40 who are making significant contributions to the fields of Pathology and Laboratory Science. If that sounds like someone you know (or maybe it’s you!) head over the nomination page and start the process. Good luck!
Month: February 2016
Microbiology Case Study: A 33 Year Old Female with Abdominal Pain
Case History:
A 33 year old African American female presents to the hospital complaining of mild abdominal pain for the past couple of days. She is 17 weeks pregnant and has a history of two prior spontaneous abortions at 15 and 16 weeks due to a shortened cervix. She is afebrile and denies any vaginal bleeding or leakage of amniotic fluid. A complete blood count reveals mild leukocytosis and anemia. On physical examination, her cervix is 2 cm dilated with bulging membranes. She is admitted for a possible cerclage placement, and an amniocentesis is performed to rule out infection prior to the procedure. The microbiology lab received 20 ml of clear, amber fluid for Gram stain and bacterial culture.



Laboratory Identification:
The Gram stain showed moderate fusiform Gram negative bacilli in a background of many acute inflammatory white blood cells. Bacterial cultures grew small, greyish-white colonies as Brucella blood agar and routine blood agar after 48 hours of incubation under anaerobic conditions at 35°C. No growth was observed on kanamycin-vancomycin laked blood (KVLB) agar. The organism was identified by MALDI-TOF as Fusobacterium nucleatum and confirmed using the Vitek anaerobic identification card.
Discussion:
Fusobacterium nucleatum is an anaerobic, Gram-negative rod that is non-spore forming. It is considered normal flora of the oral cavity and gastrointestinal & genitourinary tracts of healthy adults. F. nucleatum has been implicated in the pathogenesis of oropharyngeal infections, especially in neutropenic patients with mucositis after receiving chemotherapy or bone marrow transplant. It is an important etiologic agent in a wide spectrum of extraoral infections including bacteremia, brain abscess, osteomyelitis and infections of the genitorurinary tract, including the fetal membranes. There have been many documented cases linking infections with F. nucleatum to chorioamnionitis, preterm birth, and neonatal sepsis. The mode of transmission of F. nucleatum to the amniotic fluid can be as a result of direct extension from the vaginal tract, hematogenous spread or as recently implicated, orogenital transmission.
Given that F. nucleatum is the most common of Fusobacterium species found in clinical specimens and it’s potential to cause significant disease, early identification of the pathogen is important. It grows well on a non-selective anaerobic agar and its growth is inhibited on Bacteroides bile esculin (BBE) and kanamycin-vancomycin laked blood (KVLB) agars. After 48 hours of incubation under anaerobic conditions, the colonies measure 1-2 mm in diameter and have been noted to have a characteristic internal flecking quality that is referred to as “speckled opalescence”. On Gram stain, the fusiform cells of F. nucleatum are long (usually 5-10 µm in length), slender filaments with tapered ends and may contain spherical swellings. In regards to biochemical testing, it is indole positive and lipase negative. Disk testing for Fusobacterium spp. shows the bacteria are resistant to vancomycin and susceptible to kanamycin and colistin.
While susceptibility testing is not routinely performed for all anaerobes, testing is indicated for organisms in pure culture isolated from normally sterile sites or for those more virulent organisms for which susceptibilities cannot be predicted. In the case of Fusobacterium spp., penicillin and ampicillin resistance among isolates of has been reported due to beta-lactamase production and it is recommended that all Gram negative anaerobes have a beta-lactamase screen performed. F. nucleatum is routinely susceptible to metronidazole, clindamycin and beta-lactam beta-lactamase inhibitor combination antibiotics.
In the case of our patient, her diagnosis of F. nucleatum in the amniotic fluid specimen precluded her from obtaining a rescue cerclage procedure. She was transferred to labor and delivery for a uterine evacuation secondary to the intra-amniotic infection and delivered a non-viable fetus. She received ampicillin and gentamicin as intravenous antibiotics.

-Brooke Sims, MD, is a third year Anatomic and Clinical Pathology resident at the University of Mississippi Medical Center.

-Lisa Stempak, MD, is an Assistant Professor of Pathology at the University of Mississippi Medical Center in Jackson, MS. Currently, she oversees testing performed in both the Chemistry and Microbiology Laboratories.
Listen: Zika Virus Podcast
Dr. Diamond from the Washington University School of Medicine talked with Lab Medicine about all things Zika Virus: a brief history of the virus, modes of transmission, and the implications for laboratory professionals and pathologists.
And They Thought it was a Metastatic Tumor
A 74 year old patient presented to the emergency room with a syncopal attack. He had an underlying history of untreated adenocarcinoma of the prostate and reported of a 10 to 15 pound weight loss in the recent months.
CBC revealed pancytopenia with white cell count of 0.4 K/uL, hemoglobin of 9.9 g/dl and platelets of 22 K/uL. The clinical suspicion was widespread metastatic adenocarcinoma.
Review of peripheral smear revealed mostly lymphocytes, one blast and a large cell with very granular cytoplasm and large eccentric nucleus.
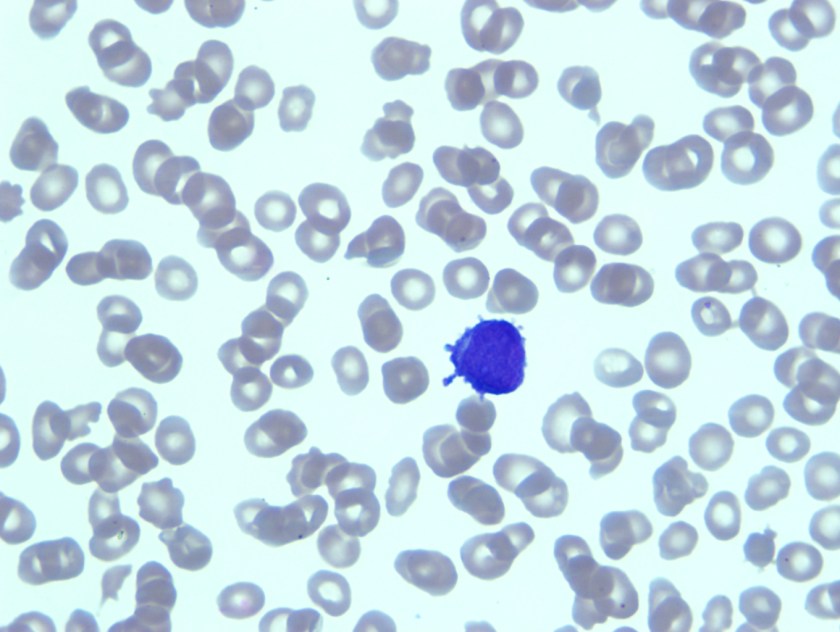
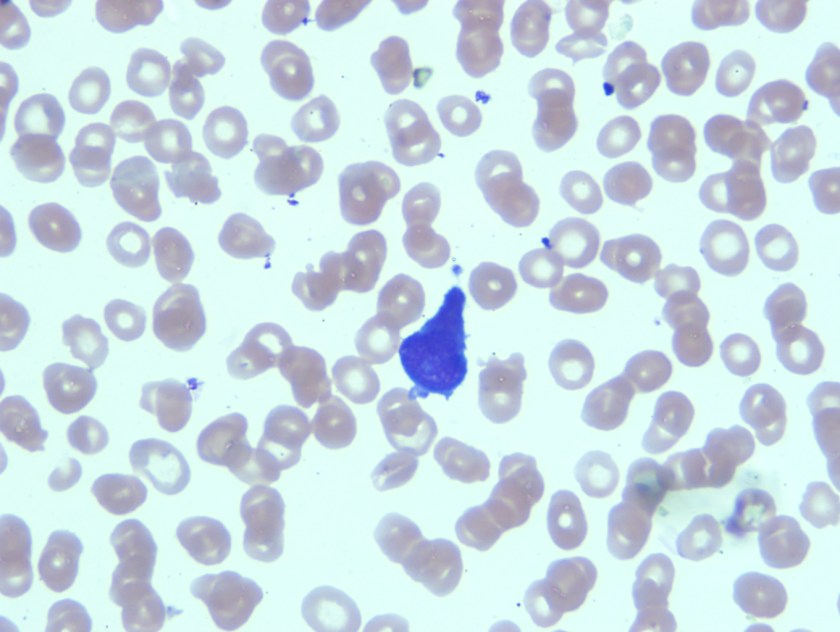
Having reviewed the peripheral smear acute leukemia, likely acute promyelocytic leukemia was considered in the differential diagnosis. As there were no dacrocytes or nucleated red blood cells that were seen on the peripheral smear, it seemed less likely that patient would have metastatic tumor. Bone marrow biopsy was recommended.
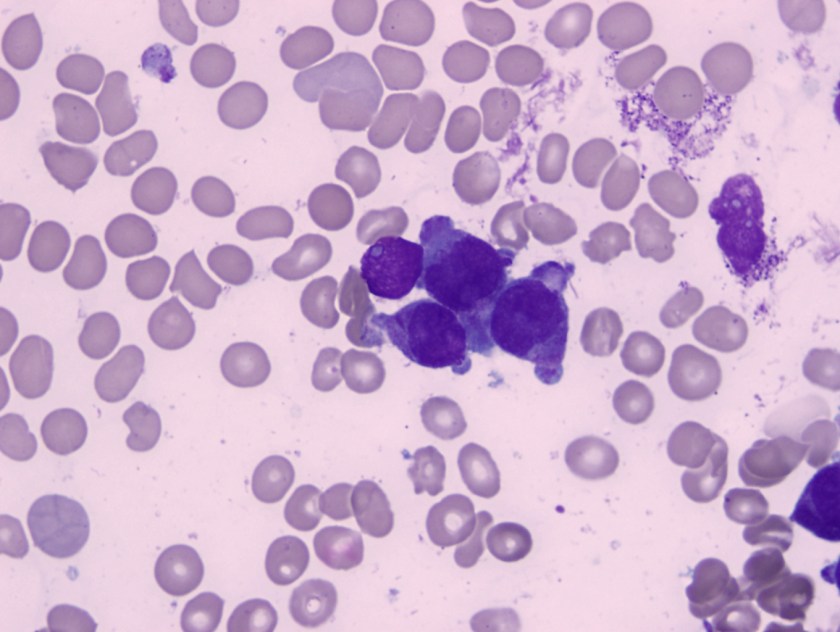
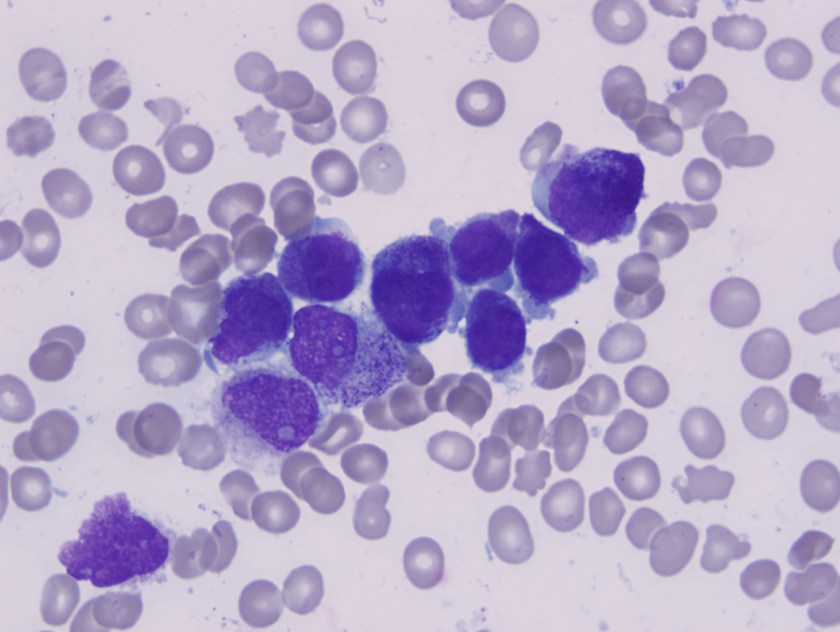
Bone marrow apsirate revealed hypercellular particles with numerous abnormal promyelocytes which were lobulated and hypergranular. Both the karytotype and FISH confirmed the presence of t(15;17).
Acute promyelocytic leukemia with t(15;17)(q22;q12);PML-RARA is an AML in which abnormal promyelocytes predominate. Typical forms are hypergranular (like this patient), although hypogranular (microgranular) forms also exist. Morphological review of the smear is the key to ordering the FISH testing for t(15;17). Often patients with APL present are at increased risk of DIC and needed to be treated on a more emergent basis.
Presence of t(15;17) defines the disease and has a significant therapeutic impact. APL has a particular sensitivity to treatment with ATRA , which acts as a differentiating agent. Prognosis of APL treated with ATRA is much more favorable than other acute myeloid leukemias.

-Neerja Vajpayee, MD, is an Associate Professor of Pathology at the SUNY Upstate Medical University, Syracuse, NY. She enjoys teaching hematology to residents, fellows and laboratory technologists.
Microbiology Case Study: An 84 Year Old Man with Weakness and Back Pain
An 84 year old man with atrial fibrillation, coronary artery disease status post bypass surgery, and type II diabetes with chronic peripheral neuropathy presented to the ED with weakness, multiple falls, back pain, chills, and confusion. He was found to have a fever of 102o F. A workup for sepsis was performed but no source of infection was identified. Blood cultures were drawn and the patient was started on broad spectrum antibiotics. Fevers, chills, and confusion improved but patient continued to have back pain. A CT scan showed a pathologic fracture of L3 vertebrae, presumed to be secondary to infection with adjacent myositis.
Multiple blood culture bottles were positive for the organism with the following Gram smear morphology and colony morphology on blood agar.
Laboratory Identification:
Gram smear showed Gram negative cocobacilli. The colonies were gray-white on blood agar, colorless on MacConkey and oxidase negative. The organism was identified as Yersinia pseudotuberculosis by MALDI-ToF and confirmed by VITEK.
Discussion:
Yersinia pseudotuberculosis is a gram negative bacillus that belongs to the Enterobacteriaceae family and therefore ferments glucose, is oxidase negative, and can reduce nitrates to nitrites. This bacterium is endemic in a wide variety of animals and is presumed to be a possible foodborne pathogen due to similarities to Yersinia enterocolitica. There have been reported cases of outbreaks associated with milk and iceberg lettuce sprayed with untreated water. Growth is optimal at 25-32o C, but can grow at 4o C. Infection is rare but typically causes enterocolitis and ileitis. Children may present with mesenteric lymphadenitis, which may mimic acute appendicitis. Sepsis is rare but is associated with those with underlying disorders such as cirrhosis, hemochromatosis, and diabetes. Reported mortality rates may be as high as 75% despite proper antibiotic treatment.
In this patient, it was determined that the most likely source of infection was consumption of raw milk. Due to presumed vertebral and paravertebral soft tissue involvement, he was treated with six weeks of IV ceftriaxone.
-Joanna Conant, MD is a 4th year anatomic and clinical pathology resident at the University of Vermont Medical Center.
-Christi Wojewoda, MD, is the Director of Clinical Microbiology at the University of Vermont Medical Center and an Assistant Professor at the University of Vermont.
The “Safety Eyes” Epiphany
The manager walked into the lab to talk to Joan about the schedule. Joan was working with Mike, an older technologist who never seemed to follow the safety regulations of the laboratory. Joan was glad her manager was coming, because today Mike was wearing mesh sneakers and he was chewing gum! She couldn’t wait to see the manager chewing him out about that. However, the manager walked quickly to Joan, asked her a question and went immediately back to the office. She never said a word to Mike.
There are several things to be said about a scenario like this. First, why doesn’t Joan say something to Mike herself? Why doesn’t she feel empowered to speak up for safety? Has anyone ever taught her how to coach her peers for safety? Second, does the manager realize how much damage was just done to the lab’s safety culture? Intended or not, her ignoring Mike’s behavior is tantamount to permitting it, and therefore promoting it.
I was a laboratory manager before I became a Lab Safety Officer. When I moved to the safety role, I was lucky to have the previous safety officer still on site to provide orientation. As we walked through the labs, my predecessor noticed several safety issues and corrected them. I was very disheartened since I didn’t notice any of them myself. I wondered if I could do the job. Not long after that, I had a “safety epiphany.” I realized I could not see those safety issues because I had not trained myself to see them. I had not yet learned how to use my “Safety Eyes.”
Since then, I have been training lab people that “Safety Eyes” is a super-power that all laboratorians have. It is a latent ability and it must be honed in order for it to be effective. In order to do that, you need to know what to look for- be aware of the lab safety regulations so you can discern between right and wrong when it occurs. Next, you need to practice. That is the most effective development method for “Safety Eyes.” Knowing where to start can be difficult, so it is best to start by focusing on one safety topic at a time.
Begin by looking at Bloodborne Pathogens issues. For example, are people wearing correct PPE? Are waste receptacles properly labeled? Are spill kits in place and not expired? Next, look at chemical hygiene issues. Are secondary containers labeled correctly? Are acids and bases stored near the floor? There are a large variety of safety items under each topic that can be checked visually in the laboratory. Move to fire safety and on to other topics. Choose one area per week to start, and over time you will become proficient in spotting safety issues with your now-powerful “Safety Eyes.”
During the first year of my role as a Lab Safety Officer, I would become angry with the lab managers who didn’t seem to support the safety program- those who would walk through their labs and not see what I considered to be obvious safety issues. Then I had to remember my days as a manager- did I pay attention to those things? No, I didn’t. In part that was because I had so many things on my plate that I was focused elsewhere- just like the manager talking to Joan about the schedule. I also did not have any Safety Eyes training, so I simply wasn’t equipped to see all of the problems.
Today, I provide Safety Eyes training to lab managers. I use photographs of safety issues- pictures taken of real issues in the lab. Using these visual aids hones their ability to see the issues the next time they walk through the lab, and it raises safety awareness for everyone. If you lead a laboratory, and if you do not focus on safety, I understand that. However, you should understand that is important to make a change- develop those Safety Eyes and advance your safety culture in a way your staff can appreciate and support.

-Dan Scungio, MT(ASCP), SLS, CQA (ASQ) has over 25 years experience as a certified medical technologist. Today he is the Laboratory Safety Officer for Sentara Healthcare, a system of seven hospitals and over 20 laboratories and draw sites in the Tidewater area of Virginia. He is also known as Dan the Lab Safety Man, a lab safety consultant, educator, and trainer.
Microbiology Case Study: Young Girl with Community-Acquired Pneumonia
A young girl presented to the emergency department with 6 days of cough, congestion, and intermittent fever up to 102°F. Respiratory viral testing, blood cultures, and a chest x-ray were ordered. The patient was found to be RSV positive and sent home with oral steroids. Two days later she presented the emergency department once again with worsening respiratory symptoms and a positive blood culture with the following organism (Figure 1).

Our patient developed a Streptococcus pneumoniae superinfection and bacteremia in conjunction with RSV pneumonia.
Identification
S. pneumoniae is a Gram-positive cocci that forms “lancet” shaped pairs on Gram stain (Figure 1). Due to pretreatment with antibiotics, our Gram stain shows some pairs, but many Gram variable chains of cocci as well. S. pneumoniae grows as alpha hemolytic colonies on 5% sheep blood, chocolate, and CAN (colistin nalidixic acid) agar in 12-18 hours, where it forms umbelicated colonies with a characteristic navel-like depression in the middle due to autolysins produced by the bacterium. Some serotypes of S. pneumoniae, primarily serotype 3, have a mucoid phenotype seen in Figure 3. S. pneumoniae is a member of the Streptococcus mitis group, but due to its pathogenic potential it has always been singled out. This is accomplished using two biochemical tests: bile solubility testing with 10% deoxycholate, which dissolves colonies of S. pneumoniae but not those of other Viridans group streptococci, and optochin disc testing, to which S. pneumoniae is sensitive while other Virdians group streptococci are resistant (Figures 2 and 3). Many molecular assays have trouble differentiating S. pneumoniae from S. mitis group due to their similarities on a nucleotide and protein level, so biochemical testing is still a mainstay of organism identification.


Clinical Significance
S. pneumoniae is known to cause a variety of clinical manifestations in children, from community acquired pneumonia and acute otitis media to bacteremia and meningitis. S. pneumoniae is also a colonizer of the upper respiratory tract; approximately 21% of children in developed countries and 90% of children in developing countries are asymptotically colonized. Due to the high rates of S. pneumoniae colonization in children, direct urine antigen testing is inappropriate, as it cannot distinguish asymptomatic carriage from invasive disease. S. pneumoniae direct antigen detection from CSF has been shown to have < 30% sensitivity and offers no benefit over a routine cytospin Gram stain.
Vaccination in children
Around 2000 the first S. pneumoniae vaccine became available. PCV7 was a heptavalent conjugate vaccine which provided protection from the 7 most common S. pneumoniae serotypes known to cause invasive disease (4, 6B, 9V, 14, 18C, 19F, and 23F). Routine vaccination of children was a huge success which reduced the incidence of invasive pneumococcal disease attributed to vaccine strains by 99%. An indirect benefit of the PCV7 vaccine was that adults >65 years of age saw a 92% decrease in invasive pneumococcal disease caused by PCV7 serotypes, despite not being vaccinated themselves, because of reduced transmission of S. pneumoniae from children to adults. Due to the selective pressure of the vaccine, non-vaccine serotypes of S. pneumoniae such as 19A subsequently became the predominant causes of invasive streptococcal disease. In 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) was FDA approved. It includes all seven S. pneumoniae serotypes contained in PCV7, plus six additional serotypes (1, 3, 5, 6A, 7F, and 19A). PCV13 provides coverage against 2/3 of all serotypes responsible for invasive pneumococcal disease in children under 5 years of age.
Follow up
The patient had an uneventful hospital stay. All subsequent blood cultures were negative and susceptibility testing found the patient’s S. pneumoniae isolate to be susceptible to penicillin, cefotaxime, and clindamycin. The patient and was discharged home after 24 hours of observation with a 7 day course of amoxicillin.
References:
- Manual of Clinical Microbiology, 11th edition
- Pediatric Red Book, 2015 Report of the Committee on Infectious Diseases, 30th edition

-Erin McElvania TeKippe, Ph.D., D(ABMM), is the Director of Clinical Microbiology at Children’s Medical Center in Dallas Texas and an Assistant Professor of Pathology and Pediatrics at University of Texas Southwestern Medical Center.
Mycobacterium chimaera Infections in Cardiac Surgery Patients
A group of physicians from the University of Iowa have started a dialogue about Mycobacterium chimaera infections in patients who have undergone cardiac surgery. It seems as though the bacteria finds its way to the patients via a heater-cooler device used during their procedure. If you’re a micro tech or a pathologist and you come across a cardiac patient who has a fever of unknown origin, night sweats, loss of energy, and failure to gain weight, M. chimaera is something to keep in mind.
You can read more about this issue in the original blog post from the Iowa infectious disease doctors, Maryn McKenna’s write up over at Nat Geo, and the CDC guidance paper.
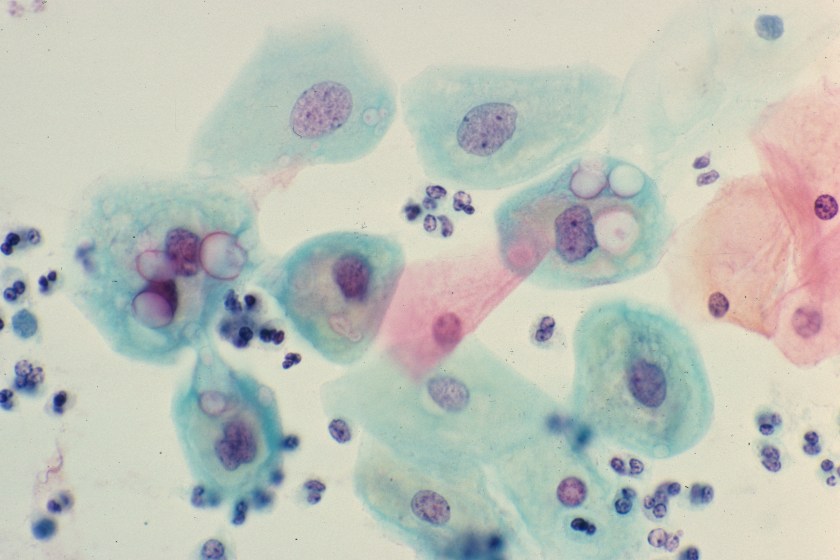
Routine Pap Smear from a 33 Year Old Woman
You are reviewing a routine Papanicoloaou smear from a 33 year-old female. She has no complaints and appears healthy. A representative field from her Pap smear is shown here. What organism is the most likely cause of the morphologic changes seen here?
- Chlamydia trachomatis
- Human papillomavirus
- Trichomonas vaginalis
- Leptothrix
- Gardnerella vaginalis
The diagnosis in this case is Chlamydial infection. Genital infection by Chlamydia trachomatis is the most common sexually transmitted disease in the world. In women, chlamydial infection of the cervix is frequently asymptomatic, as it was in this patient. If untreated, however, the infection can lead to pelvic inflammatory disease, infertility and ectopic pregnancy.
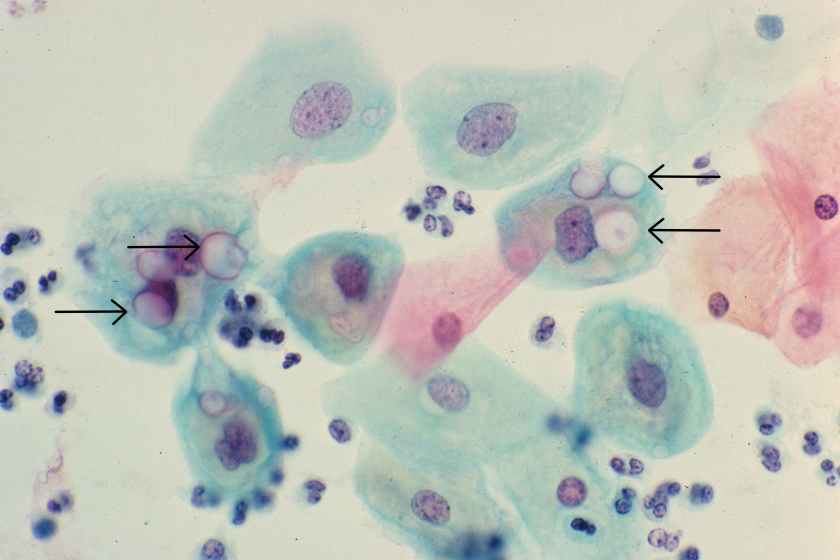
Chlamydia trachomatis is a tiny, gram-negative bacterium that exists in two different forms: the elementary body, which is the infectious form, and the reticulate body, which is the replicative form. While Chlamydial organisms are too small to be visible with a gram stain, large, glassy inclusions containing both reticulate bodies and elementary bodies are occasionally clearly visible within cells, as seen in the squamous epithelial cells in this image (see arrows).

-Kristine Krafts, MD, is an Assistant Professor of Pathology at the University of Minnesota School of Medicine and School of Dentistry and the founder of the educational website Pathology Student.



