Case History
A 3 year old girl initially presented with abdominal pain and fevers. Ultrasound identified a left kidney mass, leading to a left radical nephrectomy and excision of retroperitoneal mass. Pathology showed a Wilms’ tumor, diffuse anaplasia type. Staging uncovered multiple pulmonary metastases and involvement of a supraclavicular lymph node. She received chemotherapy and radiation without regression of disease. She then presented to the ED and was admitted for neutropenic fever. She was started on broad-spectrum antibiotics. She continued to spike fevers so an antifungal, micafungin, was added. While admitted, she developed scattered erythematous papules. Infectious disease was consulted and a skin biopsy from the left forearm was obtained.
Laboratory Results
- Bacterial cultures, blood: negative
- Fungal cultures, blood: negative
- Blastomyces urine antigen: negative
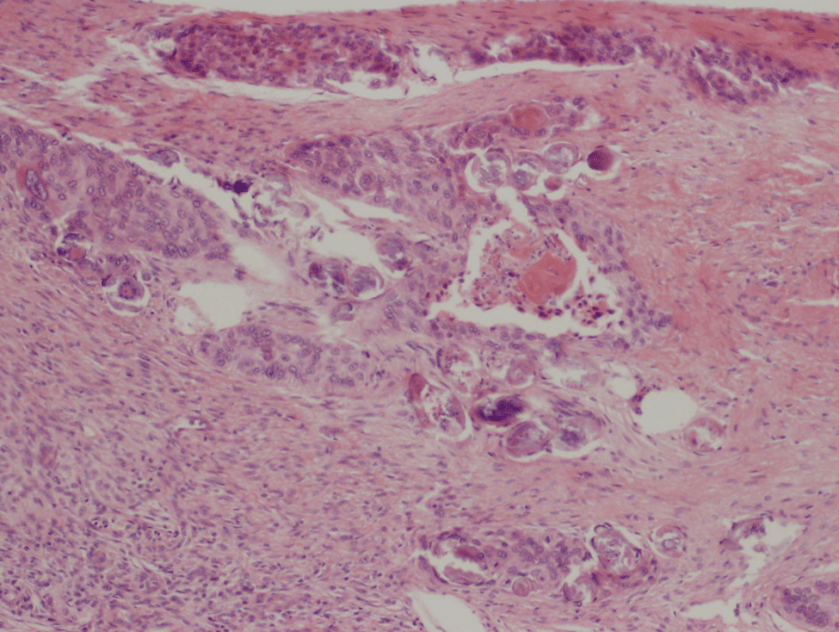
- Skin biopsy: slight epidermal hyperplasia with follicular dilatation, mild vascular ectasia, and focal erythrocyte extravasation. Negative for organisms.
- Bacterial culture, tissue: no growth.
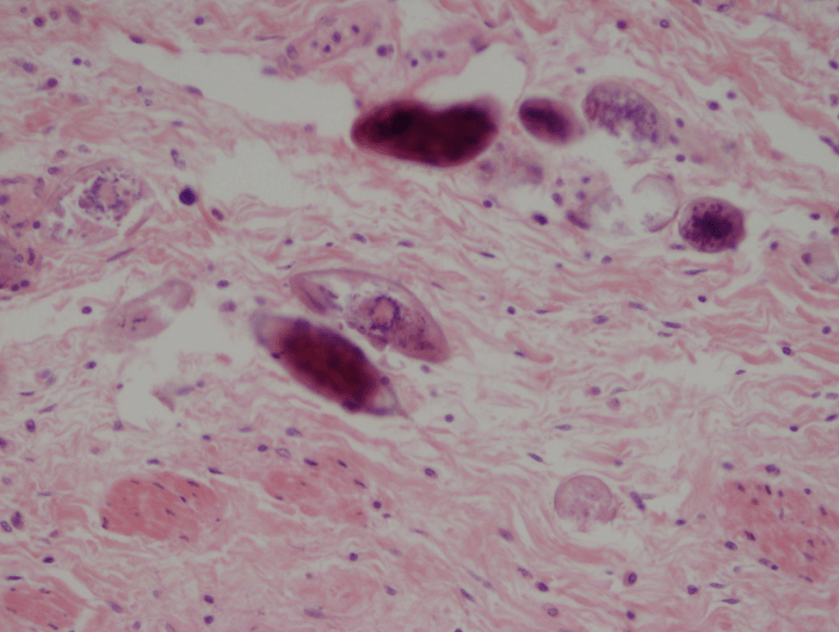
- Gram stain: rare budding yeast forms seen
- Fungal culture, tissue: no growth to date
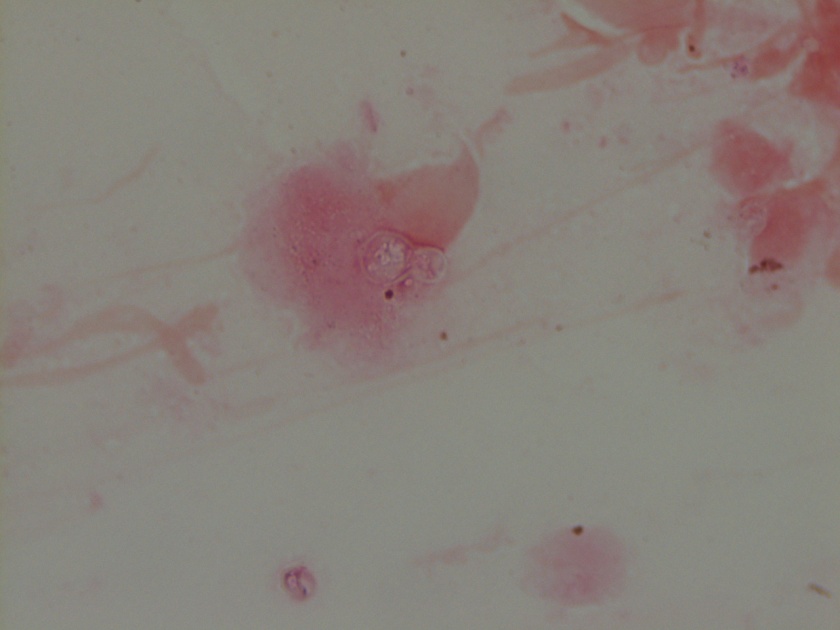
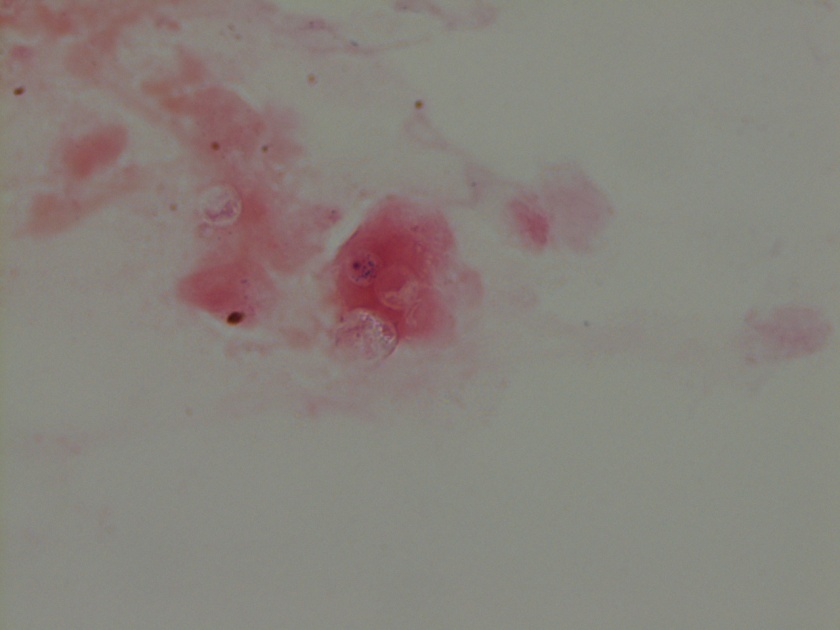
The gram smear made from skin biopsy tissue for bacterial culture displayed rare broad-based budding yeast forms, consistent with Blastomyces dermatiditis.
Discussion
Blastomyces dermatitidis is a dimorphic fungus found in moist soil and decomposing matter. It is endemic within the Mississippi and Ohio River valleys as well as the Great Lakes region and Southern United States. There are reports of infection in Africa and India. The fungus is transmitted by inhalation of as few as 10-100 conidia. Once in the lungs, the spores convert to yeast and multiply. Infection usually results in a flu-like illness with pulmonary involvement 3-15 weeks post-exposure. Hematogenous spread can further result in involvement of the skin, bone, genitourinary tract, and central nervous system.
The gold standard for diagnosis is culture or cytopathology/histology. However, the organism is a slow grower, which can take 2-4 weeks, and may fail to grow in one-third of cases. On culture at room temperatures (25-30°C), the mold form appears wrinkled and waxy and is cream to tan in color. Microscopically, they form septate hyphae with short or long conidiophores bearing small round to pear-shaped conidia (2-10 microns). This arrangement of the mold is described as a “lollipop” appearance. At 35-37°C, the fungus is a yeast (8-10 microns) with classic broad-based budding and double contoured walls.
Antigen testing is available on urine, serum, bronchoalveolar lavage fluid, and CSF. Antigen testing is more rapid, utilizing enzyme immunoassay, but has a lower sensitivity. Antigen testing is most sensitive in patients with isolated pulmonary disease. Serial urine antigen testing can be used to indicate disease regression or relapse.
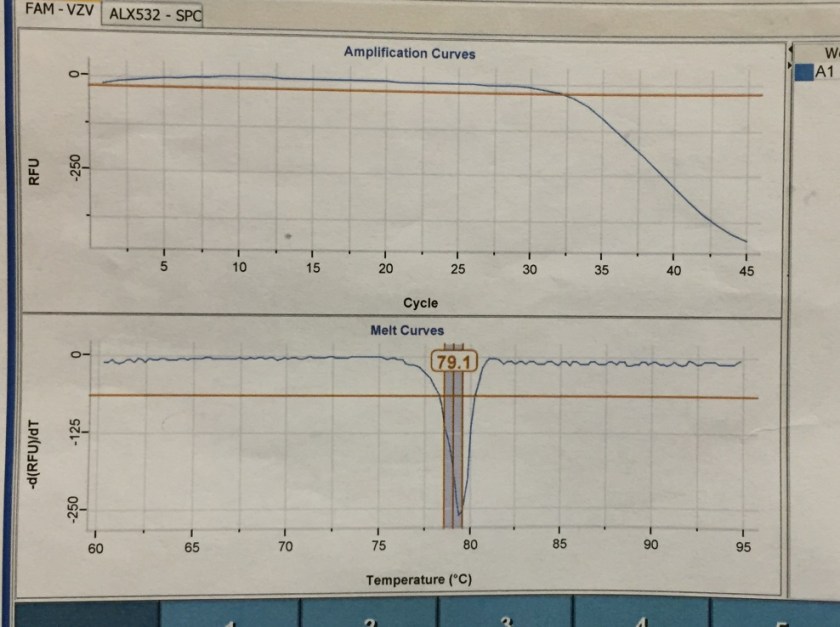
A real-time PCR assay is available for confirmation of B. dermatitidis. The probe targets the promoter region of the BAD1 gene, which encodes an adhesin molecule and virulence factor. This method can be performed in five hours, but is only available at reference laboratories.
Mild to moderate pulmonary and extrapulmonary blastomycosis can be treated with oral itraconazole. Severe cases, CNS involvement, or infection of immunosuppressed patients, pregnant women, or children require amphotericin B followed by step-down therapy with itraconazole for 6-12 months.
Upon report of the mold on gram smear, micafungin was discontinued and amphotericin B treatment initiated. Her fever and rash resolved. The patient was transitioned to oral itraconazole prior to discharge and will remain on therapy for 12 months.
REFERENCES
- https://www.cdc.gov/fungal/diseases/blastomycosis/index.html
- Frost HM, Novicki TJ. Blastomyces Antigen Detection for Diagnosis and Management of Blastomycosis. Journal of Clinical Microbiology. 2015;53(11):3660-3662. doi:10.1128/jcm.02352-15.
- Sidamonidze K, Peck MK, Perez M, et al. Real-Time PCR Assay for Identification of Blastomyces dermatitidis in Culture and in Tissue. Journal of Clinical Microbiology. 2012;50(5):1783-1786. doi:10.1128/jcm.00310-12.
- Chapman SCAW, Dismukes WE, Proia LA, et al. Clinical Practice Guidelines for the Management of Blastomycosis: 2008 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2008;46(12):1801-1812. doi:10.1086/588300.
-Prajesh Adhikari, MD is a 2nd year anatomic and clinical pathology resident at the University of Vermont Medical Center.

-Christi Wojewoda, MD, is the Director of Clinical Microbiology at the University of Vermont Medical Center and an Assistant Professor at the University of Vermont.