One of my favorite things about working in Hematology is handling those “difficult” samples. You know the ones. The one that some techs put aside to work on “later,” or they might decide it’s time to take a break when they see them coming. I love investigating and working on these interesting but perhaps uncooperative samples. At times this involves running samples in different modes, making new slides or albumin smears, and diluting samples. At other times, we investigate a delta or unusual results by checking patient diagnosis and previous results or by calling the care provider for more information and clues to help us resolve the problem.
I’m sure you’ve all seen the sayings “Without the Lab, you’re only guessing” and “Laboratory Professionals get results.” Physicians rely on the lab every day for information used to help diagnose and treat patients. Therefore, our goal is to deliver to the care provider the best possible results in a timely manner. Which means that we don’t just report results because that’s the answer the instrument gave us. With today’s instruments and middleware, we get very accurate and precise results, and about 85% or more of hematology specimens autovalidate. This is important because it leaves us time to work on those specimens with flags, and discrepancies; the ones that need a little more time and attention.
When faced with unusual or conflicting results, we first need to ask ourselves if we are dealing with a spurious sample, interfering substances or true abnormal results. Many labs today use middleware that will give the operator alerts when a sample needs to be investigated. These alerts give us suggestions as to how to handle the specimen but are usually short phrases triggered by certain values or flags and cannot be all encompassing. Operator alerts cannot tell us all the steps we may need to follow to resolve, for example, deltas, platelet clumps, abnormal scattergrams or a possible cold agglutinin. The alerts are great guidelines but it is often necessary to do more. We may need to refer to procedure manuals for SOPS or check instrument manuals or technical bulletins to decide how to handle these specimens. Sometimes we need to be detectives to report the most accurate results. We must review results with a critical eye, use all that “stuff” we learned in school, and be able to make educated decisions based on this investigation.
In my experience, one of the most common troublesome and perhaps misunderstood specimens I see is the one with a “hemoglobin (Hgb) interference” flag. An instrument flag “suspect, turbidity /Hgb interference?” is generally initiated when the MCHC is above a certain value. In our hematology lab, we see this flag when the MCHC is above 37.5 g/dL. What this is telling us is that turbidity may be present in the diluted and lysed sample. This turbidity can interfere with the Hgb detection light path and falsely increase the Hgb. Because the MCH and MCHC are calculated using the Hgb, these parameters are also affected. BUT, an MCHC >37.5 g/dL is not always something that can be or that needs to be corrected. With any parameter 95% of normal values will fall within 2SD of the mean. This means that 5% of normal healthy individuals have MCHC results <32 g/dL or >36 g/dL, and a few may have an MCHC over 37.5 g/dL. An MCHC >37.5 g/dL therefore can indicate a normal specimen, such as in a healthy young male with a Hgb at the high end of the reference range. High MCHCs can also be seen routinely in specimens from patients with spherocytosis or hemoglobinopathies such as Hgb SS, Hgb SC or Hgb C disease. In these conditions the RBCs are hyperdense due to altered surface volume and this leads to a high MCHC.
On our instrument, an MCHC >37.5 g/dL will cause a Hgb/Turbidity flag. An asterisk (*) will appear next to the Hgb, MCH and MCHC. The middleware triggers an operator alert that says “MCHC >37.5. Incubate at 37C for 30 mins. Evaluate for lipemia, icterus, hemolysis, Plasma replacement if indicated, rerun”. So, what’s the first thing to do?? Incubate? Hold on…not so fast. This is one of those instances where hematology is not just black and white. This operator alert is giving us suggestions of how to handle a specimen, but techs need to evaluate the specimen before jumping on the ‘cold’ wagon. Incubating will usually help resolve a cold agglutinin, but won’t help with a sickle cell specimen, or resolve one that’s icteric or lipemic. A grossly hemolyzed sample can give a spurious high MCHC result and, if so, needs to be recollected, not warmed. Putting a specimen that’s hemolyzed or lipemic or icteric in the heating block for 30 or more minutes would only delay reporting of results. My first case example involves a 45 year old female. The MCHC on initial run was 38.1 and the specimen gave a Hgb turbidity flag. The sample was incubated and rerun several times. After 1 hour of incubation, the MCHC was reported as 37.1 with a comment “repeated after warming for 1 hour at 37C”. In this case the patient was a known sickle cell patient. Previous results show that this patient’s MCHC is typically high and previously reported results ranged from 36.1- 37.8 g/dL. When evaluating a specimen with a high MCHC it is important to check the pattern of results. In this case the MCHC was high but the MCV was low. This does not fit the pattern for a cold agglutinin. As noted above, super dense RBCs in sickle cell patients may cause a high MCHC. This specimen was warmed, and even though the MCHC was a bit lower after warming, it would have been acceptable to report the original run MCHC. Checking patient history and previous results, and reviewing the smear for morphology would have allowed these results to be reported in a timely fashion. The operator alert does say “incubate the specimen” but it also says to evaluate. Be sure to check the MCV and MCHC along with patient history before warming specimens that don’t fit the pattern of a cold agglutinin.

The second example is from a 75 year old male. The CBC flagged Hgb turbidity with an MCHC of 45.8 g/dL. The MCHC >37.5 operator alert triggered Checking the pattern of results for the indicies, the MCHC was very high and the MCV was low. In a specimen with a low or normal MCV and a high MCHC, lipemia, icterus, abnormal proteins or severe leukocytosis can be affecting the Hgb. On evaluation, this sample’s Hgb and Hct did not meet the ‘rule of 3’. The rules of 3 are now generally recognized to be valid only for samples when the RBCs are normal, but the * here is telling us that there is an interference affecting the Hgb. In these cases it is valuable to know what the interference is so we know how to handle the specimen. By spinning down a small aliquot, (or asking chemistry!) we can investigate for lipemia or icterus. The specimen was found to be grossly lipemic. Flagging guidelines for lipemic specimens suggest diluting the specimen 1:5 and rerunning. Alternately, with severely lipemic or icteric samples, plasma replacement procedure may be necessary to correct the results. In this case, a plasma replacement was performed. After a plasma replacement, the WBC, RBC, Hct, MCV and platelet count are reported from the original run. The Hgb interference is what was causing the problem. Thus, when you correct the Hgb you must always correct any indicies that are calculated with the Hgb. The Hgb from the plasma replacement sample is used and the MCH and MCHC are recalculated. Notice that the new lower Hgb value now matches the Hct.

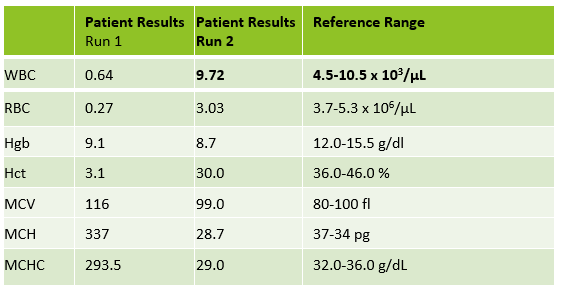
Case 3 is a sample from an 80 year old woman. This was an interesting sample because there were multiple things going on here. This patient had an initial result with a high MCHC and MCH, with decreased RBC and Hct. In this patient the initial WBC was 0.64 and the RBC was 0.31. The Hgb of 9.1 /dL was less than the Hct of 3.1 %. MCV was 116 fl and the MCHC was 293.5 g/dL! In specimens with a high MCV and high MCHC we can suspect a cold agglutinin. When the MCV is very high it is because the RBCs are going through the aperture as one big bunch and this is measured as the size of one RBC. Often the Hct is less than the Hgb. Sometimes the RBC and Hct are so low that it causes the MCV to be appear within normal range. On our instrument, a RBC count of <0.5 x106/μL will give a flag “abnormal RBC scattergram” but no other indicies related flags are generated, so we didn’t even get an operator alert to evaluate the MCHC. But, it’s clear there is something very wrong with these results. Warming the sample is used to loosen clumping of RBCs, which lowers the MCV and allows the RBCs to be counted. Make a smear to examine for RBC clumping and look at the sample tube. Many cold agglutinin samples will appear to be ‘grainy’ or have agglutination along the side of the tube. This is the time when we want to incubate the sample. To resolve a cold agglutinin, warming the sample is necessary. Sometime 30 minutes is enough, sometime they need to be incubated longer. Some cold agglutinins are so strong that after incubation a dilution or plasma replacement still needs to be done. Warming this sample did not lower the MCHC. After incubating, I diluted this sample, and also did a plasma replacement to see how results would compare. The new results matched. This sample took a bit more time than others but the cold agglutinin was resolved and we were able to report valid results.

There are other factors that can affect the Hct or Hgb and cause a high MCHC. Icteric specimens act much like lipemic ones and the Hgb can be corrected with dilution or a plasma replacement. An electrolyte balance can affect the Hct. Abnormal proteins and severe leukocytosis can affect the Hgb. Grossly hemolyzed samples can have a high MCHC. It is important to evaluate the indicies in these samples and correlate the values with previous results and patient history. What concerns me is that I have seen samples being warmed that do not match the indicies patterns for cold agglutinins. I have seen samples from sickle cell patients signed out with a comment “warmed at 37C. Possible cold agglutinin.” I have seen lipemic or icteric samples that are reported out with high MCHCs, erroneously high Hgb or parameters that are not reported at all. While warming these samples may actually lower the MCHC a bit, it still usually remains on the high side and does not give us the clean results that dilution or plasma replacement will. A little extra time looking at the indicies can give us important clues as to how to handle these samples. Doctors use our results every day to make patient care decisions. We need to make sure that we are making decisions every day to give them the best possible results so that patients can get the best care possible.

References
- Costa, B. M. B., Vellés, M. C., Viana, M. M. F. B., & Rebelo, C. I. M. (2018). Interference of cold agglutinin autoantibodies in erythrogram interpretation: a case report and literature review. Jornal Brasileiro De Patologia e MedicinaLaboratorial, 54(4). doi: 10.5935/1676-2444.20180043
- Sysmex USA. XN-Series Flagging Interpretation Guide. Document Number: 1166-LSS, Rev. 6, March 2021
- It’s not Black and White: Unraveling the puzzles of Hematology. Becky Socha MS, BB, MLS(ASCP) Mercy Medical Center, Baltimore, MD

-Becky Socha, MS, MLS(ASCP)CMBBCM graduated from Merrimack College in N. Andover, Massachusetts with a BS in Medical Technology and completed her MS in Clinical Laboratory Sciences at the University of Massachusetts, Lowell. She has worked as a Medical Technologist for over 40 years and has taught as an adjunct faculty member at Merrimack College, UMass Lowell and Stevenson University for over 20 years. She has worked in all areas of the clinical laboratory, but has a special interest in Hematology and Blood Banking. She currently works at Mercy Medical Center in Baltimore, Md. When she’s not busy being a mad scientist, she can be found outside riding her bicycle.

I had a patient with a MCHC of 50.0. When the specimen was warmed the result did not change. I looked at the chemistry results and found a protein result greater than the linearity. Once a smear was examined the rouleaux was amazing. A dilution or saline replacement resolved the issue. When I checked the historical results there where a number of results release with garbage values because no one read the procedure for MCHC trouble shooting. Live and learn.
Mark, that’s a great example to pass on to coworkers! I always try to tell techs not to assume it’s a cold agglutinin.
Hi,
I came across with a case that the original result of MCHC was 38.9 and warming did not solve the problem. Therefore, a tech spinned down the sample and it was just slightly iteric. Later, the tech performed plasma replacement and MCHC went down to 37.0. How to justify the high MCHC result was due to plasma interference?
Hi, yes, not all high MCHC’s are cold agglutinins. Warming only helps for colds. If your sample was icteric the plasma replacement looks like it did the trick. Was the Hgb corrected (does it now look more like it ‘matches’ with the Hct? Did you recalculate the indices? RBC, Hct, MCV, and Plt are reported from original run. Hgb is reported from plasma replacement sample. MCH and MCHC need to be recalculated.
Just had one of those yesterday, threw us for a loop
Hi Wing, If you look at your MCHC of 38.9, be sure to look at the other indices. Was the MCV normal , low or high? Was the Hgb consistent with the Hct, or did it look too high? Icterus can interfere with the Hgb, but generally does not affect the MCV because the RBCs are not agglutinating. Warming these specimens does not help, as you noticed. When you did the saline replacement did you correct the indices? After a plasma replacement, the WBC, RBC, Hct, MCV and platelet count are reported from the original run. The Hgb from the plasma replacement sample is used and the MCH and MCHC are recalculated. If the sample corrects this shows you that the Hgb interference was corrected. You can also check diagnosis or chemistry results to see if they correlate to a patient who may be icteric. You don’t necessarily need to ‘justify’ what was causing the interference, but note that a plasma replacement was performed. This also helps techs who get this patient’s samples at a later date know how to handle the specimen. Follow your lab’s SOPs as to the reporting, if you report a comment that says ‘icteric’ or other comments.
I would like to know when we do the dilution is it ok to keep all the results from the dilution? For example, if the specimen was icteric or lipemic could we just keep all the values from the dilution or do we only keep the hemoglobin and calculate the new mch and mchc. I know sometimes when I do a dilution all the value compare to the original. So is it safe to release the entire indices and diff from the dilution in this case?
Hi Carmen. When you do a dilution, you should always pick a parameter and multiply by the dilution factor to make sure your dilution was accurate. Then you can multipy all the results and report them. If you do a plasma replacement , then you would be correcting the hemoglobin. After a plasma replacement, the WBC, RBC, Hct, MCV and platelet count are reported from the original run. The Hgb interference is what was causing the problem, so you report the Hgb from the plasma replacement sample. When you correct the Hgb you must always correct any indicies that are calculated with the Hgb. The Hgb from the plasma replacement sample is used and the MCH and MCHC are recalculated.