Hello again! The last case study was an example of a patient with a loss of allele at two STR loci on a shared chromosome. Today, I wanted to share an interesting and unusual case that we monitor in our lab. This case explores the use of cord bloods as the source of the donor, and in this case, a double cord blood transplant.
Cord blood (CB) unit transplants can be advantageous over other donor sources, such as bone marrow or peripheral blood. The Leukemia and Lymphoma Society summarizes these advantages well, with some being their availability (CB can be prescreened/tested and then frozen for use when needed – decreasing the risk of disease transmission), less-strict HLA matching requirements, decreased graft versus host disease (GVHD) occurrence and severity, long-term storage (CB over 10 years old has been successfully transplanted), increased diversity of donors, and reduced risk of disease relapse, to name a few.2, 3
CB also has its disadvantages, some include: less stem cells for engraftment which leads to longer engraftment times, these longer engraftment times lead to longer immunological recovery and a higher risk of infection, less available clinical data relative to stem cell and bone marrow transplants (newer procedure comparatively in transplant), and no additional cells for infusions later on in treatment. Further, selecting the best cords for transplant can be challenging due to the static variables of a CB (again, there is no donor to go back and get more cells). Considering all that CB has to offer, haplo-identical transplants are preferred in the U.S. over CB transplants. 2,3,4
Before the University of Minnesota pioneered the strategy of double cord transplants, single cord transplants gave rise to a high incidence of graft failure and transplant related mortality. 2 Double cord transplants have now become standard when utilizing CB as the donor, as a single CB unit contains a small number of required and necessary cells for a successful transplant and double units help overcome the issues that this presents.
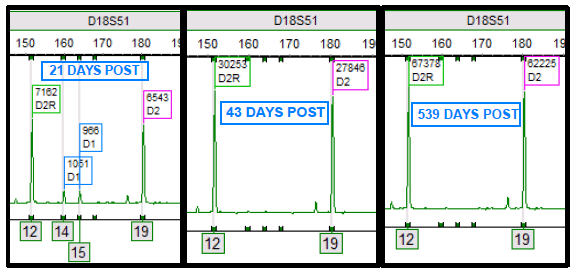
Double cord transplants are interesting and complicated for analysis purposes (and in general!). All stem cell transplants involve a dynamic process between the cells of the donor and recipient. Yet, double cords bring in another dynamic process including an additional donor.1,2 Through the chimerism monitoring process, the complexity of the engraftment process can be appreciated as one cord ultimately becomes the “winner” and the other the “loser”. In other words, one engrafts and is detectable, while the other cord fails to engraft and becomes undetectable. Figure 1 demonstrates this process, where both cords are present initially after transplant. Then, at 43 days post-transplant, a single donor cord (D2) engrafts while the other donor cord (D1) does not engraft. D1 is most likely eliminated from the host, potentially explained by multiple theories, and no longer is detectable by chimerism testing.
In the case study below, the patient was diagnosed with chronic myeloid leukemia and received a double cord transplant in 2014. One would expect, as described above, that one cord would become the “winner” while the other is rejected and becomes the “loser” and becomes undetectable. Interesting enough, this patient never achieved a status of a “winner” or “loser” cord. Rather, both remained persistent within the patient’s chimerism profile and over time have become relatively stable in their percentages.
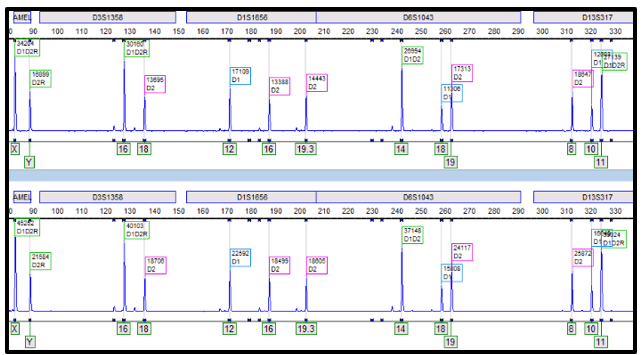
In the electropherogram below (Figure 2), alleles from both donors can be appreciated from the CD3 (top) and CD33 (bottom) lineages. Each lineage exhibits different constitutions of the donor cord percentages, where CD3 has a greater proportion of cord two than CD33; yet both lineages have a greater overall percentage of cord two than cord one. Looking at the line graph (Figure 3), the differences between the cord percentages can be further appreciated over time. It can even be noted that the cord proportions in the CD33 lineage swapped in 2017, only to swap back to favor cord two and to remain that way since. Changes of donor-recipient relative percentages occur throughout the post-transplant journey and these events are due to complex processes. Some patients become transient mixed chimerisms (who initially are mixed chimerism but later achieve total/complete chimerism), others achieve complete chimerism, and yet others may become stable mixed chimerism. It is important to note that, even in cases where complete chimerism is not achieved, disease remission can still be present.1 In this case, the patient has achieved a stable mixed chimerism status among both donor cords and, to our lab’s knowledge, is doing well clinically.

This case brings me back to a memory of my professor, who spoke briefly of this occurrence in a lecture only to quickly admit of its rarity. This is an interesting case because it represents one of those extremely uncommon instances. It is a privilege to be a part of a transplant center, like Northwestern’s, where we can witness rare and unique presentations like this. It opens up opportunities to learn and explore the complexities that transplant medicine and molecular HLA have to offer.
References
- Faraci M, Bagnasco F, Leoni M, et al. Evaluation of Chimerism Dynamics after Allogeneic Hematopoietic Stem Cell Transplantation in Children with Nonmalignant Diseases. Biol Blood Marrow Transplant. 2018;24(5):1088-1093. doi:10.1016/j.bbmt.2017.12.801
- Gutman JA, Riddell SR, McGoldrick S, Delaney C. Double unit cord blood transplantation: Who wins-and why do we care?. Chimerism. 2010;1(1):21-22. doi:10.4161/chim.1.1.12141
- Leukemia & Lymphoma Society. Transplantation Facts.https://www.lls.org/sites/default/files/file_assets/FS2_Cord_Blood_Transplantation_6_16FINAL.pdf. Published May 2016. Accessed December 15, 2020.
- Gupta AO, Wagner JE. Umbilical Cord Blood Transplants: Current Status and Evolving Therapies. Front Pediatr. 2020;8:570282. Published 2020 Oct 2. doi:10.3389/fped.2020.570282

-Ben Dahlstrom is a recent graduate of the NorthShore University HealthSystem MLS program. He currently works as a molecular technologist for Northwestern University in their transplant lab, performing HLA typing on bone marrow and solid organ transplants. His interests include microbiology, molecular, immunology, and blood bank.
