In today’s hematology lab, when physicians order a CBC with differential, they typically request a CBC with automated differential. Thus, up to 85% of our CBCs are autovalidated because they are entirely within normal range, with no instrument flags. This leaves the technologist time to spend on those slides that do need a manual review. In reviewing a slide, we evaluate the WBCs, RBCs and platelets, and must pay attention to the counts as well as morphology.
But, what do we do when we have a cell we cannot identify? When we perform a manual differential under the microscope, technologists will joke or tell stories about the legendary “skipocyte”; that cell which, while it does not look malignant or clinically significant, we still can’t decide what it is, so it’s skipped. Perhaps the best way to deal with these cells would be to get consensus from other techs or the Hematology supervisor or to request a pathology review. However, despite the fact that we are taught that there is no such thing as a skipocyte, there are times when a tech will ignore the cell, hoping they don’t see another one. But, what do we do when we see smudge cells? Are they skipocytes? What exactly are they? Do we ignore these? Are they clinically significant? Do we count them as their own category of cells? Or something else?
Firstly, what is a smudge cell? Smudge cells, or basket cells, are remnants of leukocytes. They have no cytoplasm, and sometimes all that can be seen are smashed nuclei. Smudge cells are formed from leukocytes, typically lymphocytes, that are fragile, and are destroyed or smudged in the physical process of making a smear. But, what if the instrument makes the smear? In recent years, more labs are using automated analyzers that prepare and stain blood smears. Even though these have instrument settings based on the physical characteristics of each sample, we still tend to observe these traumatic injuries to leukocytes with automated slide making. Whether we make slides manually or the instrument makes them, these fragile cells appear on the stained slide as ruptured cells called smudge cells.
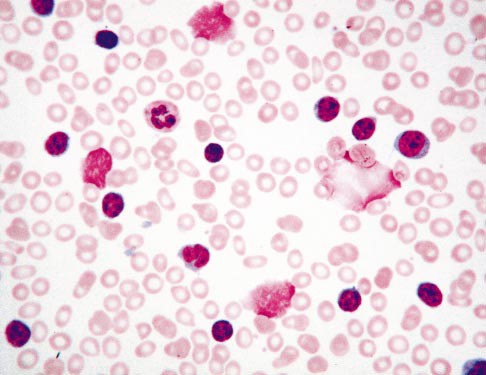
Smudge cells have also been called Gumprecht shadows, named after German scientists and researcher Ferdinand Adolph Gumprecht, who observed these on slides of patients with chronic lymphocytic leukemia (CLL). Smudge cells in patients with CLL are ruptured B-cells, but they can’t be distinguished morphologically from other disintegrated lymphocytes. We also see leukocytosis and smudge cells in viral conditions and chronic inflammatory diseases. However, the term Gumprecht shadows is reserved only for smudge cells in CLL cases.
Knowing what a smudge cell is, how do we handle them? Do we report the presence only? Do we count them? Or, do we ignore them entirely? Smudge cells are not skipocytes! For many years smudge cells were considered to be simply artifacts of slide making. More recently, studies have been conducted that show that there may be clinical significance to the number of smudge cells seen. While smudge cells are not diagnostic of CLL, it has been shown that, in newly diagnosed CLL, a larger percentage of smudge cells is a better prognostic factor. Patients with >30% smudge cells show longer times before requiring treatment and longer survival rates than patients with fewer smudge cells. These studies focused on vimentin, a protein that is important in lymphocyte cellular rigidity. Patients with low vimentin have more smudge cells and better survival rates.1,2
If we are performing a slide review, we are reviewing these slides because of some sort of instrument flag or rule trigger. There are several theories as to how smudge cells can be handled, and studies have been done to compare these theories.3 Laboratories have SOPs in place to guide technologist review and reporting, yet, I have noticed considerable variation in handling of smudge cells both within our lab and between labs. These pesky artefacts can be puzzling in both traditional (under the microscope) and digitized (CellaVision) microscopy and new technologists or unfamiliar operators can easily be misled. If we perform our manual differentials traditionally, under the microscope, we will no doubt notice the presence of smudge cells. It is important not to pass by these or consider them skipocytes. Some labs count these as their own category of cell and some labs merely report the presence of smudge cells. Other labs do not report smudge cells at all, with the exception being in known cases of CLL. In these CLL differentials, if the WBC count is very high, it may also be recommended to do a 200 cell differential. But, what happens when the manual diff doesn’t match the automated diff? The hematology analyzer will accurately count fragile cells, still intact in the specimen, and include them in the differential. If the cells then disintegrate on smear making, we see smudge cells on the slide. If we do not count these, this can affect the percentage of cell types in the differential, and potentially, in a patient with a low WBC, affect the absolute neutrophil count (ANC). If we are performing the manual differential (diff) in CellaVision, CellaVision identifies smudge cells and puts them in a separate category, but these are not reported as part of the diff. These are a ‘heads up’ to the technologist that further steps need to be taken to report out a differential. The importance of recognizing smudge cells is illustrated in Table 1 below for a patient sample with WBC 3.6 x 103/μL.
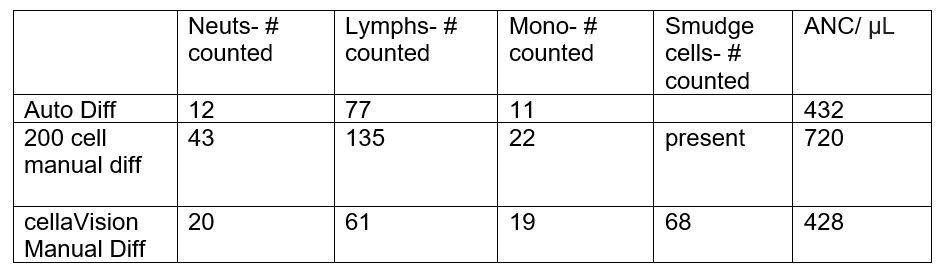
The automated differential (auto diff) in this example, with 12% neutrophils counted, has an absolute neutrophil count of 432/ μL, which is considered critical (critical <500/μL). Fragile lymphocytes are intact in the blood sample and are counted by hematology analyzers.
The 200 cell manual differential above merely notes the presence of smudge cells, but no quantifier is given. The ANC here is not critical (774/μL) and the lymph% is only 61, possibly leaving the physician to question how many smudge cells were present, and what the true lymph% may be.
In the CellavVision differential in Table 1, based on 100 WBCs counted, the total percentages of Neuts is 20%, lymphs 61% and monos 19%. If an unexperienced tech did not notice or investigate the 68 smudge cells, the manual differential (manual diff) reported from the CellaVision would be very different from the auto diff, and has an ANC of 720/μL, above the critical range.
If however, the smudge cells in CellaVision were reported as a separate category, our differential would now be based on 168 cells counted. 100 WBCs counted plus the 68 smudge cells counted = total of 168 cells counted. Our neut% is now 11.9 (20/168*100), lymph % 36.3(61/168*100), monos% 11.3 (19/168*100) and smudge cell % 40.5 (68/168*100). This ANC matches that from the auto diff. And, if we further consider that the smudge cells are lymphocytes, this brings the count to 11.9% neuts, 77% lymphs and 11.3% monos. (68 +61 = 129/168*100 = 77% lymphs) which closely matches our automated differential.
Lastly, the ‘something else’, is that we can make an albumin smear on these specimens. It has been a practice in labs to perform a manual differential on an albuminized blood smear when a certain number, defined by SOPs, of smudge cells are seen. If this is your lab procedure, it is important to recognize the presence of smudge cells on the manual differential or CellaVision differential and take the steps to make an albumin smear. Adding a drop of albumin to a few drops of the patient blood can add protein to the specimen and prevent the formation of smudge cells. Table 2 shows the manual diff on the sample in Table 1, performed on the albuminized slide. Note that this eliminates the smudge cells and corrects the diff results to match the original automated differential.

It can be seen from these examples, that the method of counting differentials with smudge cells can alter the results reported to the physician. Any of the differential methods above that count smudge cells give essentially the same results. If smudge cells are not counted, the lymphs will be under reported and the neutrophils will be over represented compared to the auto diff. Excluding smudge cells from the manual differential count or merely reporting their presence without quantification will also yield unreliable results, and then necessitates performing an albumin differential.
If we are to choose between an albumin differential and an automated differential, which studies have shown to be equivalent3, making and staining an additional smear is time consuming and can affect turnaround times. Thus, guidelines have been suggested that the first choice for handling pesky smudge cells is to review the smear and report the automated diff with a morphology comment that smudge cells are present. If automated diffs are not available, smudge cells should be counted as lymphocytes, or in a separate category4, as illustrated in Table 1. Study findings indicate that this method is sufficient for reporting a reliable manual differential on known CLL patients3. By counting smudge cells separately, however, as discussed previously, these numbers can be used in newly diagnosed cases of CLL as a prognostic indicator.
There is still debate on the value of reporting smudge cells on routine CBC smears. In most routine cases, an auto diff without quantitating smudge cells is considered sufficient. Pathologists, however, differ on whether smudge cells should be reported.
The best course of action is always to consistently follow your own lab’s SOPs, to be aware of flags, rules triggered and operator alerts with regard to smears, and to always be on the lookout for smudge cells. They are not skipocytes!
References
- Nowakowski GS, Hoyer JD, Shanafelt TD, et al. Percentage of smudge cells on routine blood smear predicts survival in chronic lymphocytic leukemia. J Clin Oncol. 2009;27(11):1844-1849.
- Amal Abd El Hamid Mohamed, Nesma Ahmed Safwat. New insights into smudge cell percentage in chronic lymphocytic Leukemia: A novel prognostic indicator of disease burden. The Egyptian Journal of Medical Human Genetics, 19 (2018) 409–415
- Gene Gulati, Vandi Ly, Guldeep Uppal, Jerald Gong, Feasibility of Counting Smudge Cells as Lymphocytes in Differential Leukocyte Counts Performed on Blood Smears of Patients With Established or Suspected Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Laboratory Medicine, Volume 48, Issue 2, May 2017, Pages 137–147, https://doi.org/10.1093/labmed/lmx002
- Denis Macdonald, MD, MBA, FRCPC, FCAP; Harold Richardson,et al. Practice Guidelines on the Reporting of Smudge Cells in the White Blood Cell Differential Count. Arch Pathol Lab Med—Vol 127, January 2003
- Luci Maria Sant’Ana Dusse; Tamiris Paula Silva, et al. Gumprecht shadows: when to use this terminology? J Bras Patol Med Lab, v. 49, n. 5, p. 320-323, 2013

-Becky Socha, MS, MLS(ASCP)CM BB CM graduated from Merrimack College in N. Andover, Massachusetts with a BS in Medical Technology and completed her MS in Clinical Laboratory Sciences at the University of Massachusetts, Lowell. She has worked as a Medical Technologist for over 30 years. She’s worked in all areas of the clinical laboratory, but has a special interest in Hematology and Blood Banking. When she’s not busy being a mad scientist, she can be found outside riding her bicycle.
