An 18 year old female with no significant past medical history experienced multiple episodes of gastrointestinal bleeding over the course of a few weeks. The most recent bout included a bloody episode that filled the toilet, for which she provided a picture for the clinician. She denies any other associated symptoms including epigastric pain, nausea, vomiting, fever, or chills. Her travel history is unknown.
Review of her history reveals an unremarkable family and social history. She has never had an incident similar to this in the past and no other family members have ever complained of similar symptoms. Review of systems was unremarkable and within normal limits. Physical exam was unremarkable. A rectal exam was performed and was noted to have brown stool that was guaiac (occult blood) positive. Non bleeding internal hemorrhoids were noted. There were no external hemorrhoids present.
Labs drawn including CBC were within normal ranges with the exception of absolute eosinophils which were at the upper limit of normal range at 0.6 x 103/µL [normal range= 0.0 – 0.6 103/µL].
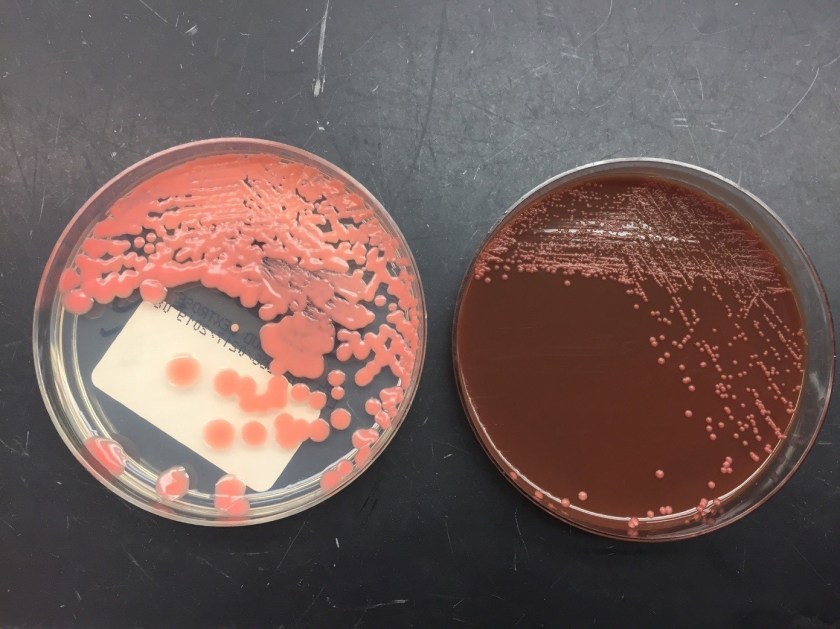
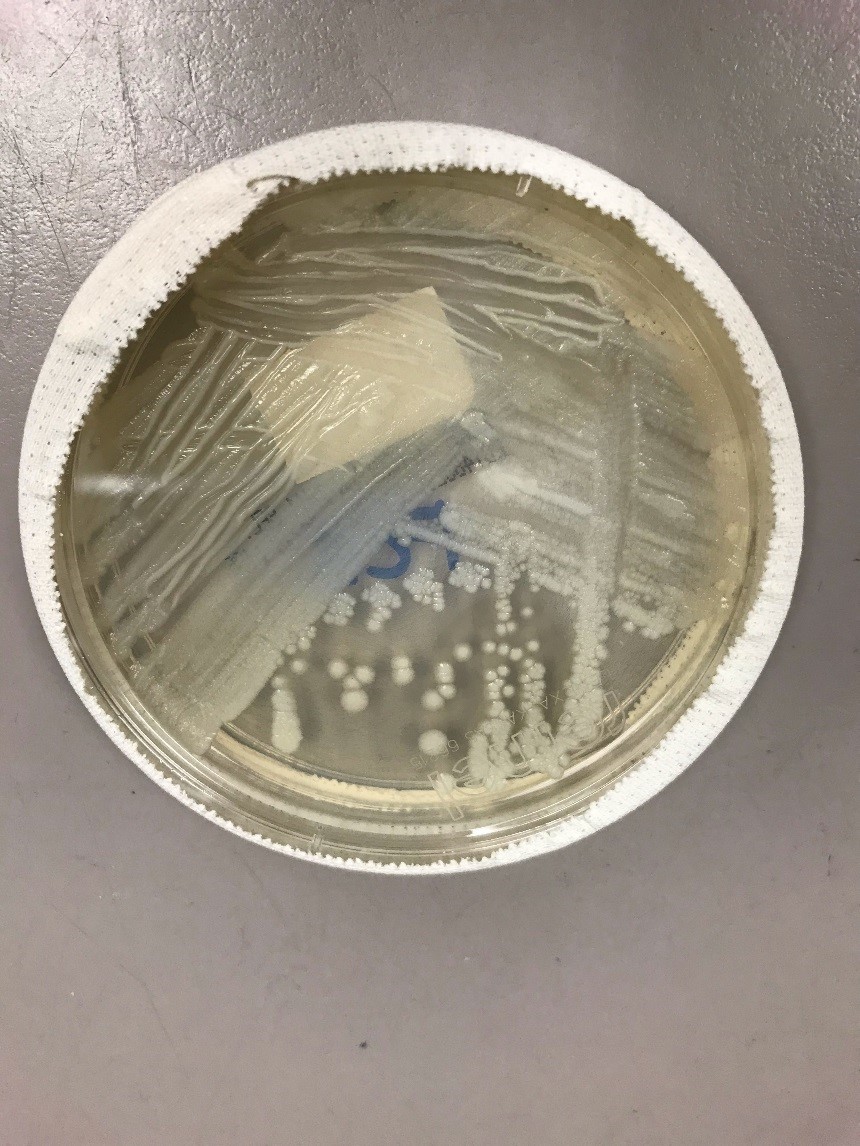
The patient had an esophagogastroduodenoscopy (EGD) to further investigate the gastrointestinal bleed. The exam was otherwise normal with exception of the ascending colon where they noted a worm on the surface of the mucosa (Image 1-2). The worm was collected and transported to microbiology for examination (Image 3-4).




Discussion
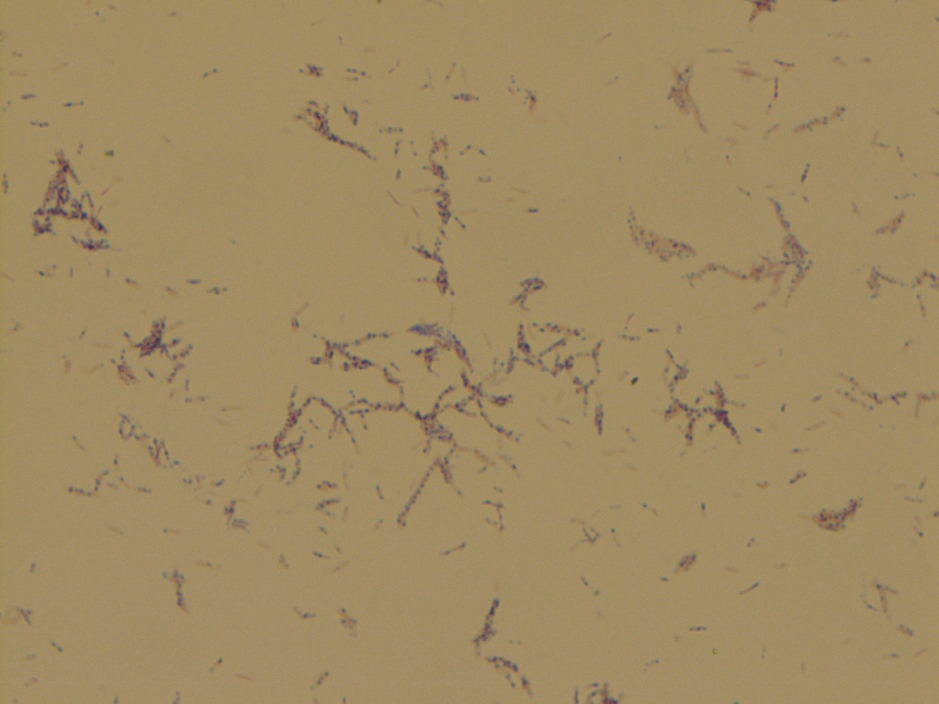
Examination of the worm and eggs revealed morphology consistent with Trichuris trichiura, or whipworm.
T. trichiura is most prevalent in warm, moist regions. The worldwide prevalence of infection is estimated to be roughly 800 million, mostly among poorer populations. Infection from T. trichiura is spread via fecal-oral route and caused by ingesting embryonated eggs. This occurs when contaminated dirt is ingested or by consumption of vegetables or fruits that have not been carefully cooked, washed or peeled.
The male and female worms both have the long whip-like structures at the anterior end. T. trichiura worms are 30-50 mm in length and the average life span is 1 year but they can live up to 10 years. The females have a straight and thick head while the males have a curly ended head. The males are typically longer the females. The eggs classically have barreled shaped, brown eggs with thick shells that measure 50-55 µm long by 22-24 µm wide. At each pole is lucent mucoid plug. The can also vary in size as noted in Image 5.
The adult female T. trichiura produces 1,000-7,000 eggs per day. The life cycle begins as unembryonated eggs passed in feces into soil (Figure 1). It takes approximately 21 days in the soil for an unembryonated egg to go through the process of embryonation to become the infective form of the parasite. Once ingested, the embryonated eggs hatch in the human intestine.


Clinically, symptoms vary depending on the worm biomass present with most infections being asymptomatic. Symptoms include cramping, weight loss, growth restriction in children, bloody stool, and anemia. It can also result in Trichuris dysentery syndrome, which is more common in children. Recurrent rectal prolapse has also been reported. Lab findings include peripheral eosinophilia. T. trichiura is treated with Albendazole for 5-7 days +/- Ivermectin. Our patient was then prescribed albendazole and is being followed in GI clinic.
References
- Centers for Disease Control and Prevention. “Laboratory Identification of Parasites of Public Health Concern: Trichuriasis”. https://www.cdc.gov/dpdx/trichuriasis/index.html
- Procop, G. W., Church, D. L., Hall, G. S., Janda, W. M., Koneman, E. W., Schreckenberger, P. C., & Woods, G. L. (2017). Koneman’s color atlas and textbook of diagnostic microbiology (Seventh edition.). Philadelphia: Wolters Kluwer Health.
-Sharif Nasr, MD, 4th year anatomic and clinical pathology resident at University of Chicago (NorthShore). Dr. Nasr has an interest in GI pathology.

-Erin McElvania, PhD, D(ABMM), is the Director of Clinical Microbiology NorthShore University Health System in Evanston, Illinois. Follow Dr. McElvania on twitter @E-McElvania.