Typically, our patients present to the endocrinology clinic after their thyroid nodules are incidentally found on staging or surveillance after being diagnosed with a primary cancer in another part of the body. Based on TI-RADS criteria, the clinician either monitors the nodule or refers the patient to radiology for a thyroid FNA. When we hear “thyroid nodule,” we rarely assume anything other than thyroid tissue. Whether the imaging favors benign or suggests a high risk of malignancy, we prepare ourselves to assess the FNA smears for follicular cells (and all the levels of atypia), colloid, macrophages, Hurthle cells, lymphocytes, etc. While we must keep an open mind, we are always caught off guard when we see anything other than thyroid-related cells. So as promised in the first edition of this post, here are four thyroid FNA cases with unsuspecting findings.
Case 1
A 47-year-old male presented with throat dysphagia and odynophagia. CT scan revealed a destructive mass within the thyroid gland with compression and invasion of the thyroid cartilage and seemed contiguous with a large pharyngeal mass, spanning approximately 8 centimeters. A follow-up PET scan noted multiple hypermetabolic thyroid masses within both lobes, direct invasion of the subglottic trachea and upper esophagus, and mediastinal lymphadenopathy.
FNA passes were obtained from the right lobe of the palpable thyroid mass.
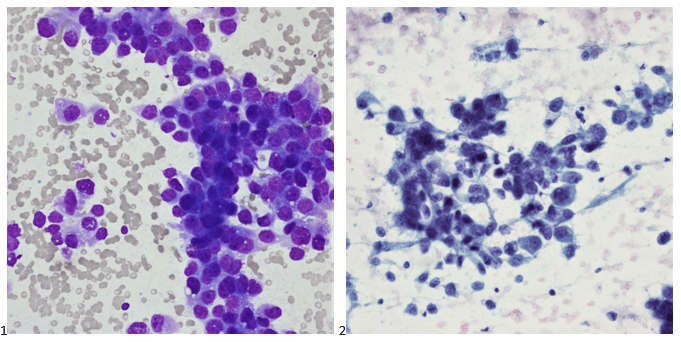
Smears (Images 1 & 2) revealed poorly differentiated neoplastic cells, follicular cells, and colloid (not visualized). No features of papillary thyroid carcinoma, medullary carcinoma, or Hurthle cell neoplasm/carcinoma were identified.
Immunohistochemical stains performed on cell block sections showed the poorly differentiated neoplastic cells to be negative for thyroglobulin, TTF-1, and calcitonin; Follicular cells, which may probably be differentiated neoplasm, were positive for thyroglobulin, TTF-1, and negative for calcitonin. Unfortunately, the scant cellularity in the cell block specimen precluded additional stains. Giant cell and spindle cell features were not identified in this specimen. Morphological features are compatible with poorly differentiated carcinoma of the thyroid gland; however, metastasis from other sites cannot be excluded.
The patient then underwent a total laryngectomy and thyroidectomy (Images 3 & 4) and level IV neck dissection, bilateral modified radical neck dissection, and tracheostomy with reconstruction performed. The patient then underwent adjuvant radiation followed by palliative re-irradiation and chemotherapy after abnormal activity was noted throughout the neck. Treatment was discontinued due to severe disease progression.
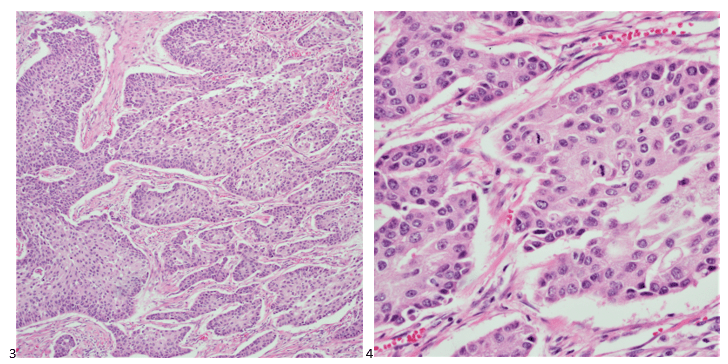
Final diagnosis: Poorly differentiated thyroid carcinoma with squamous differentiation arising in association with differentiated follicle derived carcinoma cells.
Case 2
A 68-year-old female presented with a 3.7 centimeter left lobe-filling thyroid nodule and a history of melanoma of the left anterior tibial region that was excised a decade prior. During that time, a sentinel lymph node biopsy identified microscopic metastasis. Seven years after her initial diagnosis, the patient underwent an excision of a right upper quadrant subcutaneous nodule, demonstrating metastatic melanoma. Three months after that excision, the patient had a low anterior resection of a rectosigmoid metastasis. A breast lesion was then identified five months later, and the patient underwent a mastectomy for melanoma involving the breast. Six months after her mastectomy, the patient had a segmental resection and excision of a left posterior thigh nodule, at which point she was enrolled in a clinical trial. The next month, four additional subcutaneous nodules were excised on the left thigh, calf, and arm. After 2 years of relatively stable disease, the patient underwent a partial gastrectomy, partial small bowel resection, and left lower extremity mass for recurrent melanoma. The last PET avid area to biopsy was the left-lobed thyroid nodule. Under ultrasound guidance, multiple FNA passes of the solid and hypervascular thyroid nodule. The smears (Images 5 & 6) and cell block (Image 7) featuring single cells with eccentric nuclei and prominent nuclei are presented below.
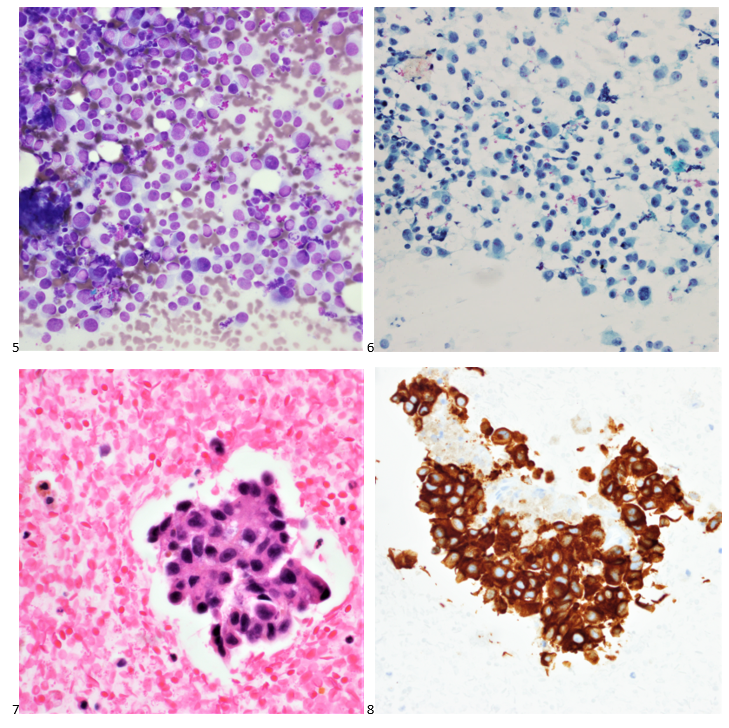
Immunostains were performed on cell block sections, and the neoplastic cells are positive for A103 (Image 8), HMB45 (scattered cells), and SOX-10, while negative for CD45, TTF-1, thyroglobulin, and calcitonin.
The patient began treatment with temozolomide and completed 33 cycles of pembrolizumab. Her most recent metastasis demonstrated extensive tumor cell necrosis, and disease progression has slowed tremendously.
Final diagnosis: Melanoma.
Case 3
After developing sudden shortness of breath and chest tightness, a 40-year-old female patient presented to the emergency department. A large mediastinal mass compressing the heart and central structures in the chest was identified on CT scan. Two thyroid nodules were also noted during that time. The patient underwent a mediastinal biopsy, which demonstrated small cell lung cancer, and the patient underwent thoracic radiation and six cycles of chemotherapy, as well as whole brain radiation. Two years later, the patient established care with endocrinology for her 1.6 centimeter solid left lobe thyroid nodule and a 1.2 cm complex thyroid nodule in the right lobe. While the right nodule was consistent with a hyperplastic nodule, the smears and cell block of the left thyroid nodule are presented below (Images 9-11).
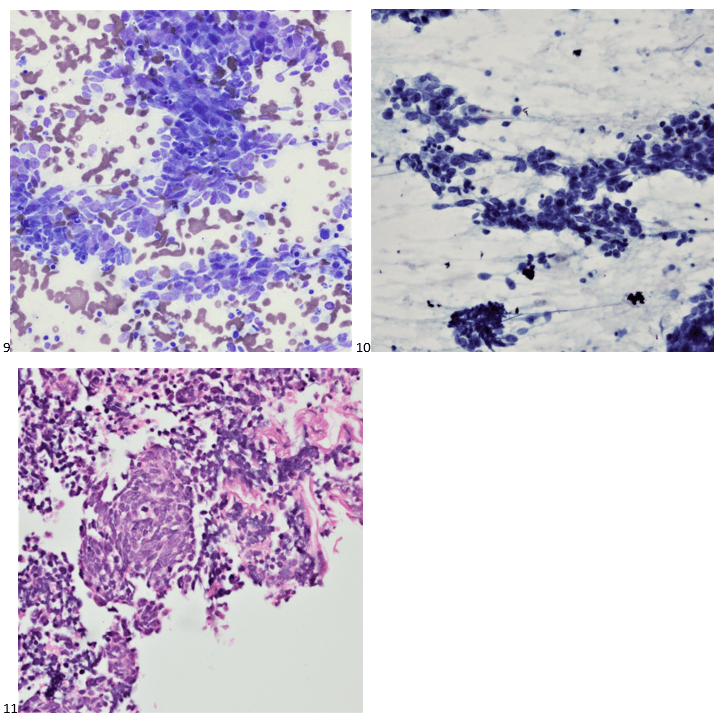
Immunohistochemical stains performed on cell block sections demonstrate the neoplastic cells were positive for TTF-1, AE1/AE3, synaptophysin, and CD56.
The patient then completed four subsequent cycles of chemotherapy with concurrent chemoradiation and is currently on active surveillance showing no evidence of disease for over 12 months.
Final diagnosis: Metastatic small cell carcinoma.
Case 4
A 59-year-old female presented to her primary care physician for gross hematuria and fatigue. Her thyroid workup demonstrated hypothyroidism on her thyroid function panel and a 2.3 centimeter solid and hypervascular thyroid nodule in the right lobe. Her urology workup revealed a 6.7 centimeter exophytic left kidney mass, and the follow-up CT scan identified a lytic lesion in the right iliac bone. The thyroid biopsy was performed in the endocrinology clinic while she was also establishing care with the urologic oncology team the same day. The smears and cell block specimen from multiple FNA passes are presented below (Images 12-14).
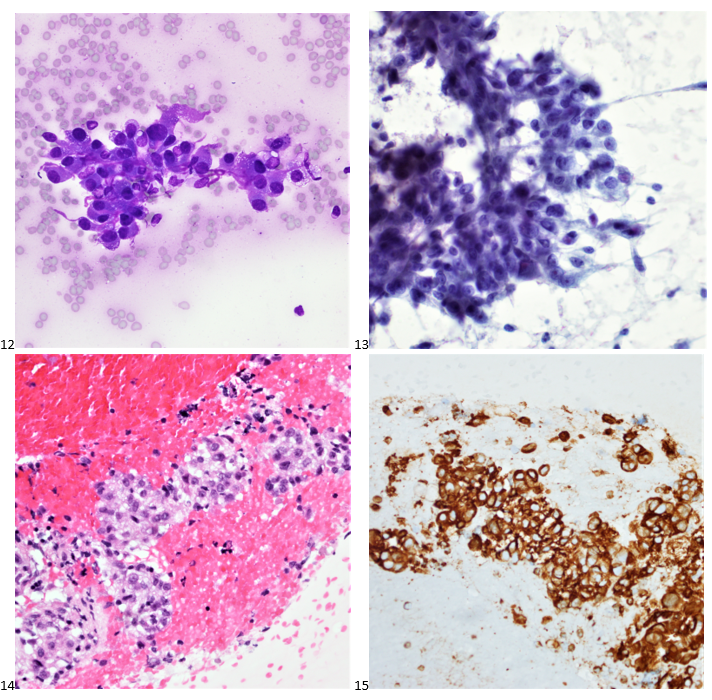
Immunocytochemical stains were performed on paraffin sections of the cell block. Tumor cells show positive staining for vimentin (Image 15), focal staining for e-cadherin, and negative staining for CK7, TTF-1, thyroglobulin, CD10, and RCC.
The patient was referred to radiology for a CT-guided biopsy of the lytic bone lesion, which demonstrated similar cells. The patient had a radical left nephrectomy, followed by sunitinib. The thyroid nodule was not responding to treatment, so they patient underwent a total thyroidectomy, which showed metastatic high-grade clear cell carcinoma with sarcomatoid progression, consistent with renal primary. In some areas, the thyroid follicles were proliferating and appear atypical, probably reactive to the metastatic carcinoma. A checkpoint inhibitor was added to the patient’s therapy, but the disease continued to progress, and the patient elected for palliative care.
Final diagnosis: Poorly differentiated carcinoma, consistent with metastatic renal cell carcinoma.
That’s a wrap! Stay tuned for the next series of cytology case studies!

-Taryn Waraksa-Deutsch, MS, SCT(ASCP)CM, CT(IAC), has worked as a cytotechnologist at Fox Chase Cancer Center, in Philadelphia, Pennsylvania, since earning her master’s degree from Thomas Jefferson University in 2014. She is an ASCP board-certified Specialist in Cytotechnology with an additional certification by the International Academy of Cytology (IAC). She is also a 2020 ASCP 40 Under Forty Honoree.
