Case History
A 50 year old female with a complex medical history consisting of lymphoma, diabetes mellitus (type II), sarcoidosis, congestive heart failure, chronic renal failure (stage 3), and pancytopenia presented to the emergency department with shortness of breath, cough, fever. She was found to be positive for SARS-CoV-2 and was transferred to the ICU due to hypoxic respiratory failure. She was treated for sepsis and respiratory failure, but her status continued to decline. The patient had multiple admissions due to COVID-19 in the past, received remdesivir and was on corticosteroid therapy due to the interstitial lung disease from last year. Initial evaluation included complete blood count which revealed anemia (hemoglobin=8.7 mg/dl), leukocytosis (WBC = 21,900/mcl), lymphopenia (910/mcl) and thrombocytopenia (Plt = 27000/mcl). The patient was treated with broad antibiotics and additional steroids. Additional tests revealed hyperproteinemia and hypoalbuminemia. Chest x-ray showed worsening infiltrates in lungs and chest CT scan revealed left apical hydropneumothorax, loculated left pleural effusion, pneumomediastinum, and chest wall subcutaneous emphysema. Lung biopsy revealed necrosis. Histopathology examination revealed broad, branching hyphae with sporulation in lung tissue biopsy and bronchoalveolar lavage. Respiratory cultures of lung biopsy and BAL grew rapidly and lactophenol cotton blue tape preps showed broad hyphae with round sporangium and rhizoids between the stolons. The patient was diagnosed with mucormycosis, infection with Rhizomucor, and was treated with Amphotericin B. Surgical debridement of the tissue was not possible due to her declining condition. She passed away after 5 days.
Discussion
The term mucormycoses refers to infections caused by the Zygomycetes which is further separated into Mucorales and Entomophthorales. Some of the members of Mucorales are Rhizopus spp., Mucor spp., Lichtheimia (Absidia) spp., Syncephalastrum spp., and Rhizomucor spp.1,2 These organisms live in soil, dung, and vegetative matter. Infection is usually acquired by inhalation/ingestion of their spores or direct inoculation and contamination of wounds. The mold can invade the walls of the blood vessels causing angioinvasion and often results in dissemination of mycotic thrombi and development of systemic infection. Zygomycetes are most commonly known for causing rhinocerebral, pulmonary, cutaneous, and disseminated disease. Infections with Zygomycetes most commonly occur as opportunistic infections in immunocompromised hosts. Risk factors include diabetes, those with acidosis, neutropenia, and sustained immunosuppression such as after transplantation.
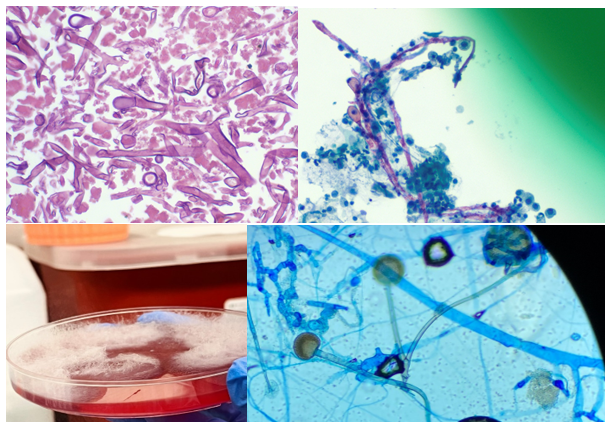
Zygomycetes grow very fast (within 48 to 72 hrs.) and is often called a “lid lifter”. The colonies have a wooly mycelium and can be described as cotton candy-like. Lactophenol tape preps of the mold would reveal broad hyphae, aseptate or pauciseptate, ribbon-like hyphae with irregular width. At the tip of the sporangiophore, there is a sack-like structure called a sporangia with contains all the spores. Fungal elements and hyphae seen on tissue biopsies from patients with mucormycosis typically have near right angle branching (usually >40o) broad, non-septate hyphae. In contrary, those with aspergillosis show acute angle branching (usually <45o) with narrow, septate hyphae.3
Genus-level identification can be achieved by microscopic morphology. Rhizomucor is an intermediate between Rhizopus and Mucor. Rhizoids found in Rhizomucor are few in number and are located on stolons, between the sporangiophores, as opposed to Rhizopus where the rhizoids are often seen directly at the nodes and Mucor which does not produce rhizoids. Sporangia (40-80 µm in diameter) are brown in color and round in shape. Apophysis is absent, which allows for differentiation from Lichtheimia (Absidia) where apophysis can be seen.4 The genus Rhizomucor includes three species: Rhizomucor pusillus, Rhizomucor miehei, and Rhizomucor tauricus.5
Treatment of mucormycosis consists of antifungal and surgical therapy. Amphotericin B is the most commonly used antifungal agent. Liposomal amphotericin B has also been successfully used in some cases with zygomycosis due to Rhizomucor.6 Early diagnosis and treatment are crucial and mortality rate is high.7 Of note, Zygomycetes are intrinsically resistant to voriconazole.
References
- Rippon J W. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes. Philadelphia, Pa: Saunders; 1974. Mucormycosis; pp. 430–447.
- Scholer H J, Müller E. Beziehungen zwischen biochemischer Leistung und Morphologie bei Pilzen aus der Familie der Mucoraceen. Pathol Microbiol. 1966;29:730–741.
- Mohindra S., Mohindra S., Gupta, R., Bakshi, J., Gupta, S. K. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. doi: 10.1111/j.1439-0507.2007.01364.x.
- de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of Clinical Fungi, 2nd ed, vol. 1. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands)
- Schipper M A A. On the genera Rhizomucor and Parasitella. Stud Mycol. 1978;17:53–71.
- Bjorkholm, M., G. Runarsson, F. Celsing, M. Kalin, B. Petrini, and P. Engervall. 2001. Liposomal amphotericin B and surgery in the successful treatment of invasive pulmonary mucormycosis in a patient with acute T- lymphoblastic leukemia. Scand J Infec Dis. 33:316-319.
- Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin Microbiol Rev. 13:236-301.

-Maryam Mehdipour Dalivand, MD is a Pathology Resident (PGY-1) at The George Washington University Hospital. She is pursuing AP/CP training.

-Rebecca Yee, PhD, D(ABMM), M(ASCP)CM is the Chief of Microbiology, Director of Clinical Microbiology and Molecular Microbiology Laboratory at the George Washington University Hospital. Her interests include bacteriology, antimicrobial resistance, and development of infectious disease diagnostics.
