Case History
The microbiology laboratory received a tissue specimen from a male patient in his 60s. The tissue was biopsied from the patient’s hand during surgery at an outside hospital, but no further clinical information was available. No organisms were observed on the Gram stain, fungal smear, or fluorochrome stain and no white blood cells were observed on the Gram stain. No organisms were recovered from the aerobic or anaerobic bacterial culture or fungal culture.
After 20 days of acid-fast bacilli (AFB) culture, yellow colonies grew on the Lowenstein Jensen (LJ) media and the 7H10 media (Image 1) and acid-fast bacilli were noted on the Kinyoun stain prepared from the colonies (Image 2).


The ordering physician was contacted regarding the positive Kinyoun stain. Upon hearing that acid-fast bacilli grew in culture, the physician shared that he has a high suspicion for Mycobacterium marinum as this patient developed a wound infection after he cut his hand with a fish bone while cooking. MALDI-TOF identified this organism as Mycobacterium marinum, confirming the astute physician’s hypothesis.
Mycobacterium marinum
Mycobacterium marinum is a slow growing non-tuberculous mycobacterium (NTM) that causes skin and soft tissue infections. M. marinum can be found in both fresh and saltwater environments and is commonly associated with contact with fish, fish tanks, marine shells, and non-chlorinated swimming pools, earning the names “swimming pool granuloma” and “fish tank granuloma.” Infections are normally localized to the skin and occur following trauma, with the limbs and extremities most often infected. Nodules or ulcers around the site of inoculation are most common—these are typically single nodules or ulcers but may spread along the lymphatic vessels to regional lymph nodes, similar to sporotrichosis. Rare manifestations of M. marinum include osteomyelitis and disseminated disease, with non-cutaneous manifestations occurring predominately in immunocompromised populations. M. marinum is a slowly progressing disease and the patient may not present to their provider until months after the initial trauma, often complicating the link to water or fish exposures. M. marinum, while it can be transmitted from fish to human, is not transmitted person-to-person and thus does not pose an infection control risk.
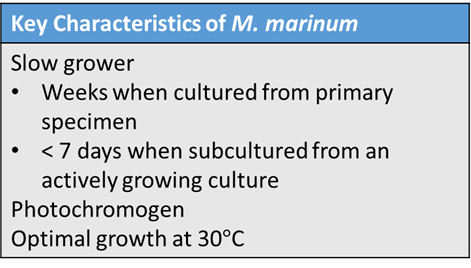
AFB cultures are typically incubated at 37 degrees C. However, M. marinum grows optimally at 30C with scant or no growth at 37
degrees C. Thus, when skin and soft tissue samples are received for AFB culture, specimens should be held at both 37 degrees C and 30 degrees C to support growth of most NTM and to facilitate the growth of M. marinum, respectively. Recovery of M. marinum in culture from primary specimen takes several weeks to grow, but subculture may take less than 7 days.
M. marinum can be identified by phenotypic characterization, molecular identification, or mass spectrometry. M. marinum is a photochromogen, meaning that colonies possess a yellow pigment when grown in light and no pigment when grown in the dark. This is characteristic of group I of Runyon’s classification. Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF), which has been used for years in most clinical microbiology laboratories for the identification of bacteria and yeast, can be used to identify Mycobacteria to the complex or species level. While the processing of Mycobacteria for MALDI-TOF is more laborious than for bacterial identification, the use of MALDI-TOF for identification of Mycobacteria is increasing in use. Whole genome sequencing can be performed on pure isolates for molecular identification, although this is more expensive and less common than MALDI-TOF or phenotypic identification.
A thorough history identifying risk factors for M. marinum should be conducted by the physician. Given the length of time for growth, clinical suspicion due to water or fish exposures can guide treatment before the organism grows in culture. In addition to providing a diagnosis and guiding clinical care, a history of M. marinum infection is also critical to note for latent tuberculosis screening as M. marinum can cross react with tuberculin skin testing and interferon gamma release assays.
References
- Aubry A, Mougari F, Reibel F, Cambau E. Mycobacterium marinum. Microbiol Spectr. 2017 Apr;5(2). doi: 10.1128/microbiolspec.TNMI7-0038-2016. PMID: 28387180.
- Wu TS, Chiu CH, Yang CH, Leu HS, Huang CT, Chen YC, Wu TL, Chang PY, Su LH, Kuo AJ, Chia JH, Lu CC, Lai HC. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7(7):e41296. doi: 10.1371/journal.pone.0041296. Epub 2012 Jul 20. PMID: 22911774; PMCID: PMC3401166.
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, Rodríguez-Morales AJ, Villamil-Gómez WE, Gotuzzo E, Bonifaz A. Cutaneous Mycobacterial Infections. Clin Microbiol Rev. 2018 Nov 14;32(1):e00069-18. doi: 10.1128/CMR.00069-18. PMID: 30429139; PMCID: PMC6302357.

Paige M.K. Larkin, PhD, D(ABMM), M(ASCP)CM is the Director of Molecular Microbiology and Associate Director of Clinical Microbiology at NorthShore University HealthSystem in Evanston, IL. Her interests include mycology, mycobacteriology, point-of-care testing, and molecular diagnostics, especially next generation sequencing.
