Case History
A 53 year old male presented to the emergency department with a one-day history of malaise, bilateral flank pain and decreased urine output. His past medical history was notable for decompensated cirrhosis due to alcohol use disorder complicated by esophageal varices and gastric ulcers, peritoneal ascites, several recent episodes of upper GI bleeding, monoclonal gammopathy of unknown significance, and remote prostate cancer status post prostatectomy. Oral history indicated the patient was actively drinking shortly before presentation and had recently consumed oysters on the half shell, broiled shrimp, and crab meat. While being seen in the emergency department, the patient quickly progressed to septic shock.
Admission laboratory studies demonstrated severe metabolic acidosis, pancytopenia, and acute renal failure. Initial CT abdomen/pelvis demonstrated cirrhotic liver changes, varices, distal esophageal wall thickening, bilateral perinephric fat stranding extending into the pelvis and perivesical fat. The patient was admitted to the MICU for intubation/mechanical ventilation and administration of multiple pressors, as well as empiric meropenem, vancomycin and micafungin given concern for septic shock.Initial blood cultures drawn in the ED flagged positive with gram negative rods (both aerobic and anaerobic bottles) less than 24 hours after collection.
Laboratory Identification
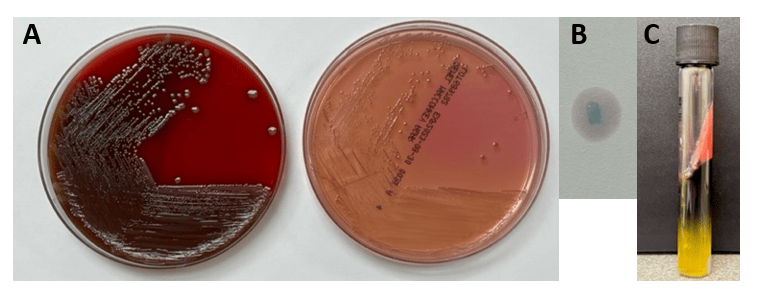
Blood cultures were processed for culture workup and molecular identification. No identification was able to be established using a commercial multiplex PCR-based molecular panel on the positive blood culture broth. Subsequent growth of the organism on MacConkey agar 18 hours later revealed non-lactose fermenting colonies (Image 1A). The organism was oxidase-negative, positive for catalase and indole (Image 1B) and exhibited hydrogen sulfide production when inoculated on triple sugar iron (TSI) media. The organism was definitively identified as Edwardsiella tarda by MALDI-TOF MS and was broadly susceptible to all antibiotic tested including beta-lactams and fluoroquinolones.
Discussion
Edwardsiella tarda is an infrequently isolated member of the Enterobacterales which is most often associated with gastroenteritis. Reports of additional presentations include peritonitis, intra-abdominal abscess, and wound infections are increasing.1 Bacteremia is rare, and can lead to cholangitis, cholecystitis, and liver abscess via hematogenous spread. Patients of advanced age (>65 years) and those with hepatobiliary diseases including liver cirrhosis and alcohol abuse and iron storage disorders are at increased risk for extraintestinal disease..2 Colonization of the gastrointestinal tract is believed to be a precipitating even leading to bacteremia. Cases of gastroenteritis are usually self-limiting, although ciprofloxacin or trimethoprim/sulfamethoxazole can be utilized in cases of prolonged duration.5 Prompt and accurate diagnosis of E. tarda bacteremia is important as this presentation is associated with an elevated mortality rate,4 particularly among those with liver disease.
As members of the Enterobacterales, Edwardsiella sp. share common biochemical features of other members of the order including being catalase-positive and oxidase-negative. Oxidase negativity aids in the distinction between E. tarda and Aeromonas/Plesiomonas, both species which are also associated with aquatic environments and cause gastrointestinal infections.5 E. tarda is non-lactose fermenting and reduces sulfur containing amino acids to hydrogen sulfide. This can result in confusion with members of the genus Salmonella4 as colonies exhibit similar appearances on medias including Hektoen Enteric agar and Xylose-lysine deoxycholate agar formulated for recovery and presumptive identification of Salmonella from gastrointestinal specimens. Importantly, E. tarda is also indole-positive which will biochemically differentiate these two genera.
E. tarda is associated with freshwater or brackish aquatic environments. This organism is a well-known pathogen in aquaculture industries causing serious infections in fish and significant economic loss. As such, vaccines and prophylactic antibiotics are utilized to prevent E. tarda infection among aquatic animals, and diagnostic approaches including specialized multiplex PCRs are available. Human infection is often associated with consumption of contaminated fish and seafood, and diagnosis is almost exclusively dependent on microbiological culture by contrast. Clinical multiplex molecular panels for both gastrointestinal and bloodstream infections lack the ability to detect E. tarda. In this patient’s case, recent consumption of seafood serves as the most likely event leading to E. tarda gastrointestinal colonization and is consistent with previously reported cases of E. tarda bacteremia.3 The patient was treated with meropenem eventually narrowed to piperacillin/tazobactam and blood cultures cleared. He was weaned off pressors and extubated. Unfortunately, the patient decompensated over the course of three weeks due to worsening shock and acidosis. He was moved to comfort care and expired soon thereafter.
References
- Hirai et. al. 2015. Edwardsiella tarda bacteremia. A rare but fatal water- and foodborne infection: Review of the literature and clinical cases from a single centre. Can. J. Infect. Dis. Med. Microbiol. 26(6): 313-318.
- Hasegawa M., and Sanmoto, Y. 2024. Recurrent cholangitis and bacteremia due to Edwardsiella tarda: a case report. Oxford Med. Case Rep. Volume 2024, Issue 1, January 2024, omad148, https://doi.org/10.1093/omcr/omad148
- An et. al. 2023. Case Report: Disseminated Edwardsiella tarda infection in an immunocompromised patient. Front. Cell. Infect. Microbiol. 20 November 2023.
- Janda, J.M, and Abbott, S.L. 1993. Infections Associated with the genus Edwardsiella: the Role of Edwardsiella tarda in human disease. Clin. Infect. Dis. Oct;17(4):742-8.
- Janda, J.M and Abbott, S.L. 1999. Unusual Food-Borne Pathogens: Listeria monocytogenes, Aeromonas, Plesiomonas, and Edwardsiella species. Clin. Lab. Med. 19(3):553-582.

-Andrew Clark, PhD, D(ABMM) is an Assistant Professor at UT Southwestern Medical Center in the Department of Pathology, and Associate Director of the Clements University Hospital microbiology laboratory. He completed a CPEP-accredited postdoctoral fellowship in Medical and Public Health Microbiology at National Institutes of Health, and is interested in antimicrobial susceptibility and anaerobe pathophysiology.
-Francesca Lee, MD, is an associate professor in the Departments of Pathology and Internal Medicine (Infectious Diseases) at UT Southwestern Medical Center
