Case History
An 83 year old female with history of type 2 diabetes presented to the emergency room (ER) with two month history of dysuria and diarrhea. Upon admission, Clostridiodes difficile (C. difficile ) GDH/TOX with reflex to nucleic acid amplification test (NAAT), urine culture, and 2 sets of blood culture specimens were collected for testing. C. difficile test was positive for GDH and Toxin A/B production. Urine culture was positive with 50,000 – 99,000 colony forming units (CFU)/mL of Klebsiella pneumoniae. Patient was administered ceftriaxone, metronidazole and vancomycin and discharged three days post admission. On day of discharge, blood cultures were documented as “no growth at 3 days”. The patient visited her primary care physician the day after discharge. Two days later, the primary care office was notified of a corrected report. The patient’s blood culture collected on day of admission was being corrected from no growth to a positive with growth of Fusobacterium nucleatum. The patient was contacted to check on her status and medication compliance was verified. Patient continued to demonstrate symptom improvement during follow up at physician’s office 10 days post discharge.
What happened here?
The blood culture was collected at an acute care hospital. The system microbiology laboratory policy for a first blood culture positive that is read as no organisms seen (NOS) from an acute care hospital laboratory, is to inoculate appropriate media and prepare three smears. Gram stain is to be performed on one of the smears and if organisms are not seen, a stained and unstained smear, along with inoculated media, are to be sent to the core microbiology laboratory (core lab). The bottle is then to be reloaded on the blood culture instrument.
The policy for a second positive alert from the same NOS bottle is to inoculate appropriate media and prepare 3 cytospin smears. Gram stain is to be performed on one of the cyto-smears and if organisms are still not seen, a stained and unstained smear are to be sent to the core lab along with the positive bottle and inoculated media. The core lab is to use the unstained cytospin smear to make and read an acridine orange (AO) stain.
In this patient’s case, the anaerobic blood culture bottle from one set flagged as positive on day 4 of incubation. The gram stain was read as NOS and inoculated media and smears were sent to the core lab. The bottle was reloaded on the instrument and the bottle alerted as positive a second time that day. The cytospin gram stain was read as NOS and inoculated media, smears, and blood culture bottle were sent to the core lab. At the core lab, a new unspun smear was made from the bottle. AO was performed on this smear and read as NOS.
In our laboratory, if a gram stain result is not entered for a blood culture, it will auto verify as a negative blood culture every 24 hours with a final negative result at 5 days. Both blood culture sets on this patient resulted as no growth at 5 days.
At the 48 hour (6 days post-collection) culture read, BAP, CHOC, and MAC demonstrated no growth, but the CDC ANA had growth. The isolate was setup for MALDI identification and resulted as 99.9% Fusobacterium nucleatum.
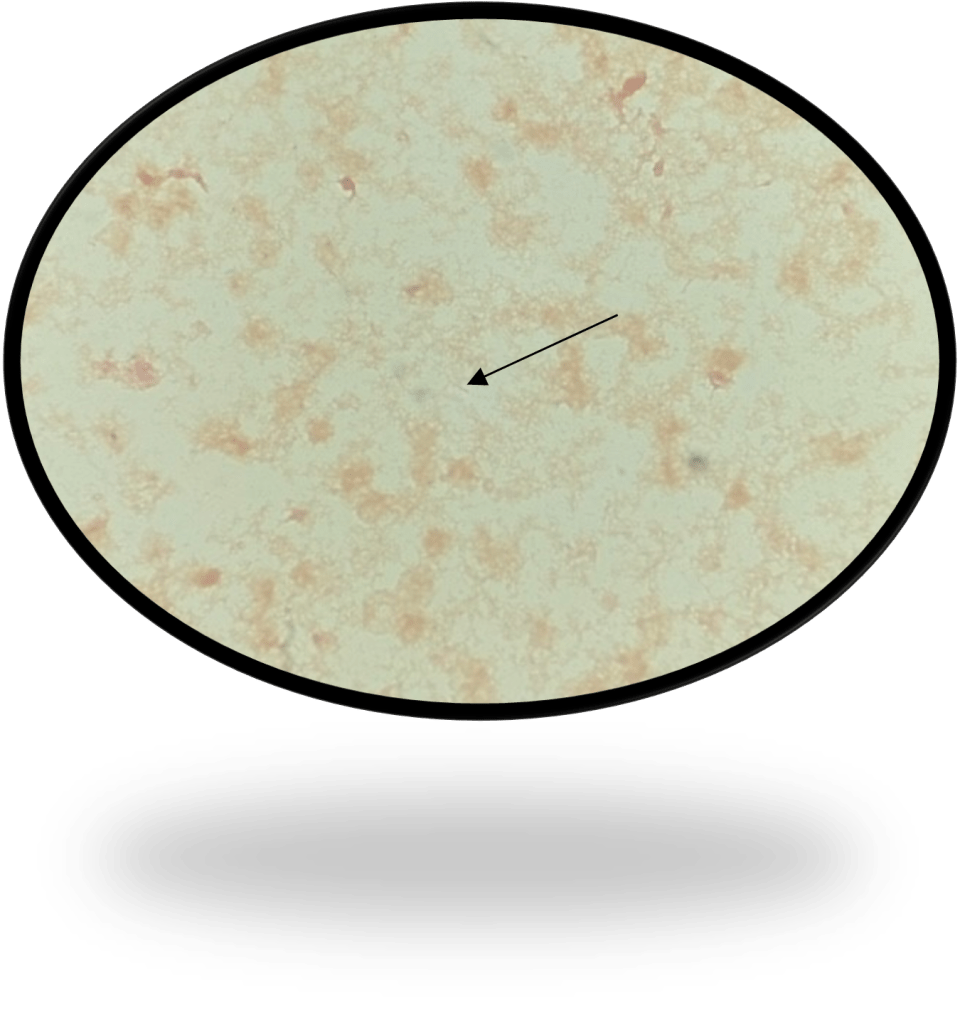
Per protocol, a review of smears was performed on this discrepant smear-culture. The unstained cyto-smear sent by the acute care laboratory had not been stained with AO and read per protocol. Rather, as was mentioned above a Gram stain was made on a new unspun smear and read as NOS. Reviewing this AO stain during the investigation revealed long thin fluorescing rods with tapered ends (Fig 1)
The cyto-smear that had been sent after the second positive alert, but did not get stained and read was also stained with AO and long thin fluorescing rods with tapered ends were observed (Fig 2).
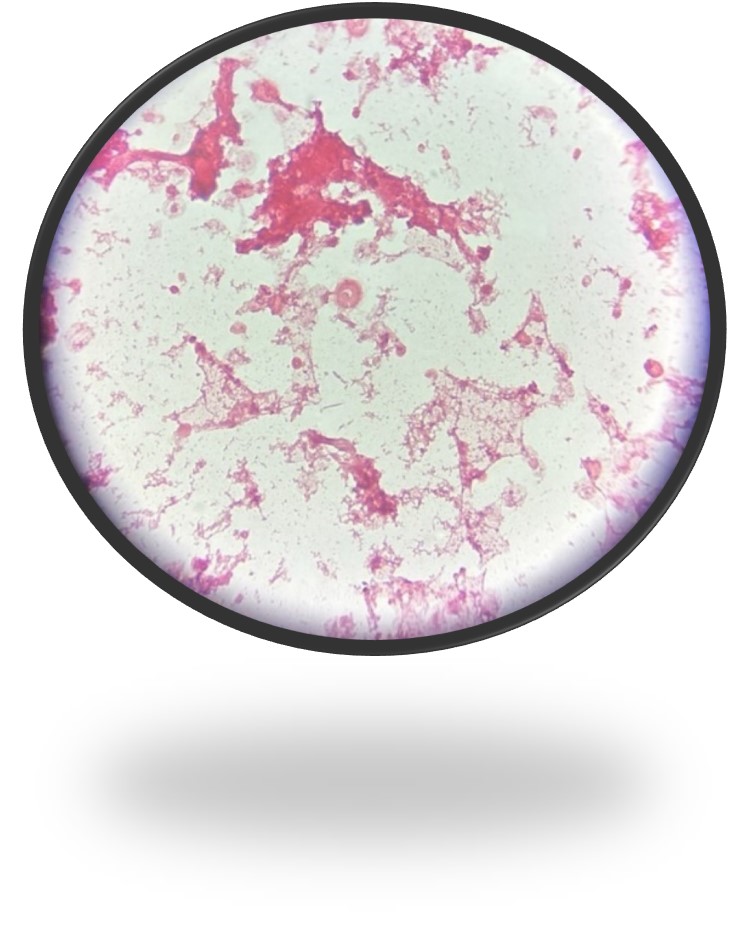
At this point in the investigation, the unspun gram stain from the 1st positive alert that was sent over from the acute care hospital laboratory was also reviewed. Knowing that the bottle was positive for F. nucleatum the technologist shared that she eventually saw rare long thin gram-negative rods with pointed ends that matched what was seen in the AO (Fig 3). As part of the investigation, safranin was added for an additional 10 minutes revealing the organism more clearly (Fig 4).


Discussion
Fusobacterium is an obligate anaerobic gram-negative rod that gram stains as a light staining thin rod with pointed ends. Fusobacterium are found in oropharyngeal flora and are commonly seen in oral biofilms. It is a primary pathogen seen in peri-implantitis, root canal infections, dentoalveolar abscesses and spreading odontogenic infections. It can also be a pathogen seen in abscesses in various parts of the body and seen in the blood stream.
Due to the staining characteristics of Fusobacterium species, they often blend into the background of gram stains from positive blood cultures. As a result, the miss in gram stain and delay in culture growth combined with the late detection of Fusobacterium on blood culture instrumentation for these fastidious organisms can result in a false negative report that is only caught after the organism grows from the original NOS bottle.
Safranin is a secondary stain or counterstain, utilized in the Gram stain, that will stain the colorless gram negative bacteria pink or red. Legionella, Brucella melitensis, Legionella and Campylobacter species are all reported to be enhanced with safranin left on for 2 minutes and it is recommended if anaerobes are suspected and not seen to leave on for 3-5 minutes or use basic fuchsin in order to enhance the morphology of these organisms. For this reason, some laboratories routinely use basic fuchsin as the counterstain.
Acridine orange (AO) is a fluorochromatic dye which binds to nucleic acids of microorganisms and human cells. Acridine orange is a fluorochrome stain that binds to the nucleic acid of cells and bacteria. RNA and single‐stranded DNA will appear orange and double‐stranded DNA found in human cells, with the exception of red blood cells, will appear yellow or yellow‐green, when visualized under UV light. Therefore, bacteria and fungi stain bright orange while epithelial, white blood cells, and background debris will appear pale green to yellow. To name a few, some common applications for AO include routine screening of normally sterile body fluids and rule‐out of Gram‐positive microorganisms versus crystal violet stain precipitate. Of important note, while not able to differentiate the actual organism, it does work for detecting Mycoplasma and Ureaplasma.
Conclusion
Blind subculturing of NOS gram stains from positive blood cultures, longer staining with safranin, and AO stains are beneficial to be added to the micro lab armamentarium. They are especially beneficial when added into the protocol for processing sterile body site specimens in which organisms, that stain lightly and blend in the background, may be suspected. For further review, Special Media or Stains for Fastidious and Infrequently Encountered Organisms can be found in the Clinical Microbiology Procedures Handbook 5th edition.
References
Kononen E, Nagy E, Conrads G. 2023. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and Other Anaerobic Gram-Negative Rods. Manual of Clinical Microbiology 13th edition. ASM Press, Washington, DC.
Tille PM. Bailey & Scott Diagnostics Microbiology, 14th ed., St. Louise, Mosby, Inc., 2017.
Dallas SD and Harrington A. 2023. 3.2 Staining Procedures. Clinical Microbiology Procedures Handbook 5th edition. ASM Press, Washington, DC.

-Jennifer Tedrick MLS(ASCP), Technical Specialist

-Maureen Bythrow, M(ASCP), Microbiology Manager

-Frances Valencia-Shelton, PhD, D(ABMM), SM(ASCP)CM is the Clinical Infectious Diagnostics Director for the Baptist Health System in Jacksonville, FL. She is actively engaged in the Jacksonville Area Microbiology Society and the American Society for Microbiology. Her interests include defining and utilizing clinical best-practice for testing and reporting. She is equally interested in learning with and educating others in the field of clinical microbiology.
