Case Description
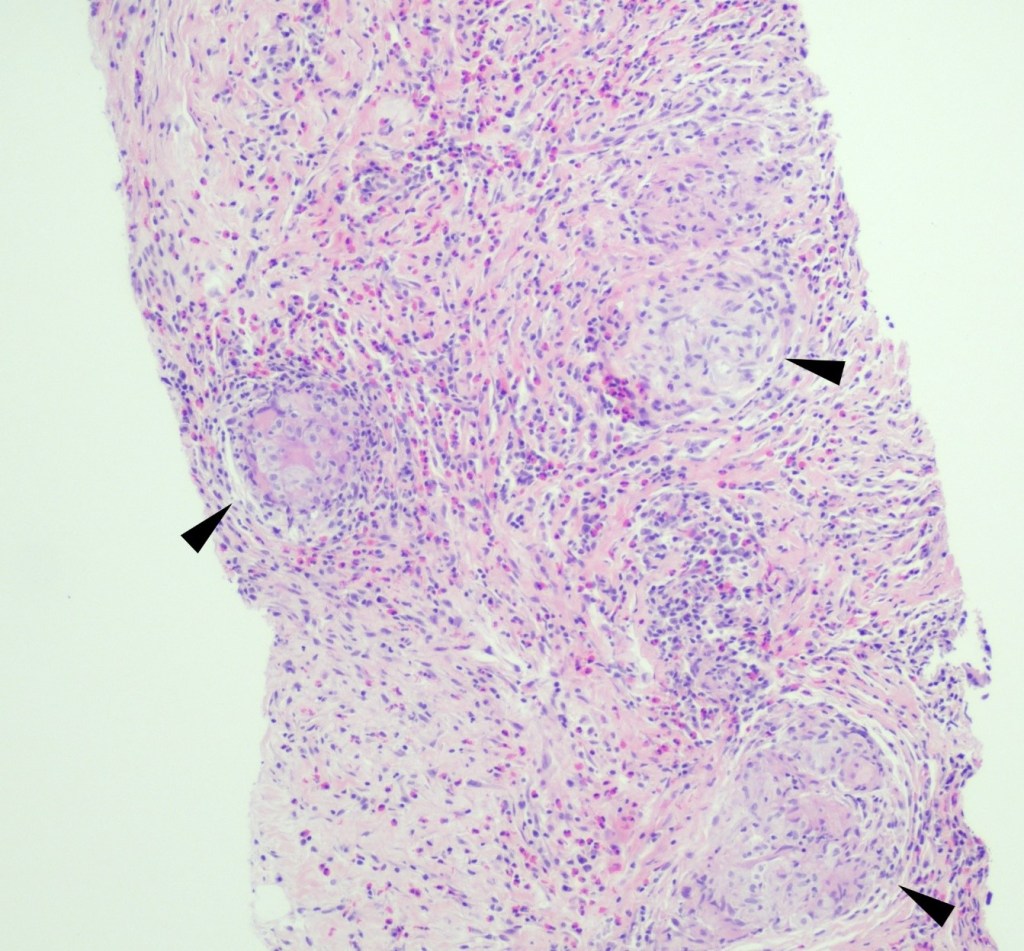
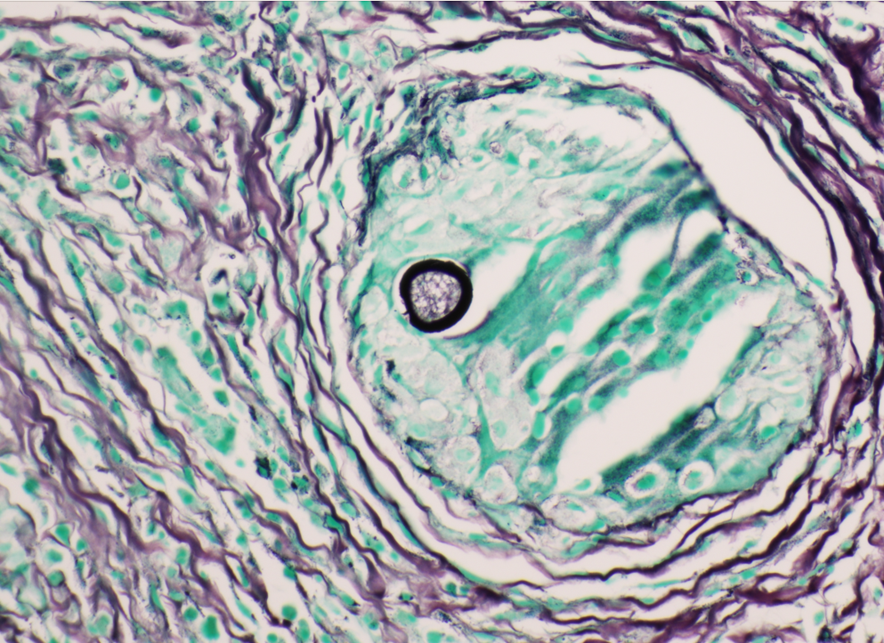
A 59 year old female presented with a one-month history of generalized abdominal pain, difficulty breathing, and early satiety. She was previously seen at an external facility and found to have liver hypointensity and abdominal distension which were concerning for liver cancer. Social history included travel to Sacramento and California deserts for hiking in the last few months. CT of the abdomen and pelvis demonstrated extensive peritoneal carcinomatosis with no primary etiology and a right hepatic lobe focus. MRI revealed diffuse omental caking and peritoneal thickening consistent with peritoneal carcinomatosis. Hepatic lesions also seen on MRI were consistent with hemangiomas. Laboratory evaluation was positive for Hepatitis B virus infection along with elevated liver enzymes (AST and ALT), CA-125, and normal levels of CEA. Paracentesis was performed removing 3 liters of yellow, cloudy fluid. Cytology demonstrated benign mesothelial cells, macrophages, and mixed inflammatory cells with a lymphocytic predominance in the peritoneal fluid. No malignant cells were identified. Initial aerobic, anaerobic, mycobacterial, and fungal cultures were negative. Serum Cryptococcus neoformans antigen testing was also negative. Ultrasound-guided core biopsy of the omental mass was performed; however, tissue was not sent for culture. Histopathology revealed granulomatous inflammation with eosinophilic infiltrate (Figure 1). A singular spherule was highlighted by GMS stain (Figure 2). Fungal serology via complement fixation for Coccidioides was positive (titer: 1:8) while Blastomyces, Histoplasma, and Aspergillus serologies were negative. The patient was subsequently managed on fluconazole.
Discussion
Coccidioidomycosis is caused by the thermally dimorphic fungus Coccidioides that is endemic to the Southwestern United States, Mexico, and some regions in Central and South America. In endemic regions, farm workers, construction workers, military personnel, archaeologists, excavators, inmates, and correctional facility officers are most affected.1 Individuals with significant exposure to the outdoors in endemic regions may be disproportionately affected. Multiple species of Coccidioides can cause disease and geographic distribution is changing2; however, the clinical presentation and approach to treatment of coccidioidomycosis are the same.
The pathophysiology of Coccidioides infection requires inhalation of arthroconidia which may result in pulmonary disease. Once inhaled, the organism forms an endospore-filled spherule in host tissue. Mature spherules rupture and disseminate endospores into surrounding tissue, which can then undergo additional replication cycles within the host. Symptomatic pulmonary disease appears to be related to fungal burden and usually presents with fever, fatigue, cough, pleurisy, and dyspnea, or less commonly peripheral eosinophilia and high erythrocyte sedimentation rate.3 Extrapulmonary disease, also known as disseminated coccidioidomycosis, is observed in <1% of cases. Extrapulmonary spread may develop in the meninges, skin, bone, or joints via hematogenous or lymphatic spread. Rarer sites of disease include the peritoneum, glandular tissue, visceral organs, bone marrow, reproductive organs, the pericardium, kidney, and the bladder.3,4
The gold standard for diagnosis of coccidioidomycosis is culture; however, histopathology may yield definitive diagnosis, especially in cases where culture was not obtained. Following histopathological examination, polymerase chain reaction (PCR) from a variety of sources may also confirm the diagnosis, especially in cases without supporting clinical or laboratory data to suggest coccidioidomycosis.5 When culture is performed, Coccidioides is recovered in its environmental, or mold form. This phase is highly infectious and can pose a significant biosafety hazard; therefore, laboratory personnel should be appropriately notified when Coccidioides is suspected, especially since Coccidioides sp. can grow on solid media within the timeframe of conventional bacterial cultures.3 Serology can be used as an adjuvant test methodology in suspected cases. Serology may be performed on blood samples, CSF, joint, or pleural fluid and measured by immunodiffusion (ID), enzyme-linked immunoassay (EIA), or complement fixation (CF). ID uses gelatin as the medium in which antibodies are placed in one well and Coccidioides antigens are placed in another. Where the two meet through diffusion, a band will form from precipitated immune complexes. ID tests for both IgG and IgM antibodies.6 EIA, in contrast, uses enzyme-labeled antibodies to detect patientantibody complexed with bound antigen. CF incubates Coccidioides antigens with patient serum that has been heated (to inactivate endogenous complement). Following incubation, fresh, non-human serum containing complement is added. If immune complexes have formed between patient antibodies and the antigens, then the fresh serum complement will affix to those complexes and indicator red blood cells will remain intact when added. A positive result will have a suspension of intact red blood cells that can be titered. A negative test will result in lysed red blood cells. CF tests for IgG antibodies.6 Coccidioides antigen assays may be used to test the urine, blood, and CSF of patients with extrapulmonary disease. In immunocompromised hosts, serology may be negative, and coccidioidal antigen assay may be performed. Clinical management commonly involves long-term antifungal therapy with or without surgical intervention for high-risk individuals.4
References
- “Epidemiology.” UC Davis Center for Valley Fever, https://health.ucdavis.edu/valley-fever/about-valley-fever/epidemiology/index.html. Accessed 6 July 2023.
- Mazi PB, Sahrmann JM, Olsen MA, Coler-Reilly A, Rauseo AM, Pullen M, Zuniga-Moya JC, Powderly WG, Spec A. The Geographic Distribution of Dimorphic Mycoses in the United States for the Modern Era. Clin Infect Dis. 2023 Apr 3;76(7):1295-1301. doi: 10.1093/cid/ciac882. PMID: 36366776; PMCID: PMC10319749.
- Proia L. Chapter 28: The Dimorphic Mycoses. In: Spec A, Escota G, Chrisler C, Davies B., eds. Comprehensive Review of Infectious Diseases. > ed. Elsevier; 2020: 411-423.
- Johnson RH, Sharma R, Kuran R, Fong I, Heidari A. Coccidioidomycosis: a review. J Investig Med. 2021;69(2):316-323. doi:10.1136/jim-2020-001655.
- Binnicker MJ, Buckwalter SP, Eisberner JJ, Stewart RA, McCullough AE, Wohlfiel SL, Wengenack NL. Detection of Coccidioides species in clinical specimens by real-time PCR. J Clin Microbiol. 2007 Jan;45(1):173-8. doi: 10.1128/JCM.01776-06.
- “Explanation of Coccidioides Diagnostic Testing.” UC Davis Center for Valley Faver. https://health.ucdavis.edu/valley-fever/about-valley-fever/coccidioides-diagnostic-testing/index.html. Accessed October 26, 2023.
-Evelyn Ilori, MD, PhD is a third-year AP/CP resident at University of Texas Southwestern Medical Center in Dallas, Texas.

-Andrew Clark, PhD, D(ABMM) is an Assistant Professor at UT Southwestern Medical Center in the Department of Pathology, and Associate Director of the Clements University Hospital microbiology laboratory. He completed a CPEP-accredited postdoctoral fellowship in Medical and Public Health Microbiology at National Institutes of Health, and is interested in antimicrobial susceptibility and anaerobe pathophysiology.

-Clare McCormick-Baw, MD, PhD is an Assistant Professor of Clinical Microbiology at UT Southwestern in Dallas, Texas. She has a passion for teaching about laboratory medicine in general and the best uses of the microbiology lab in particular.
